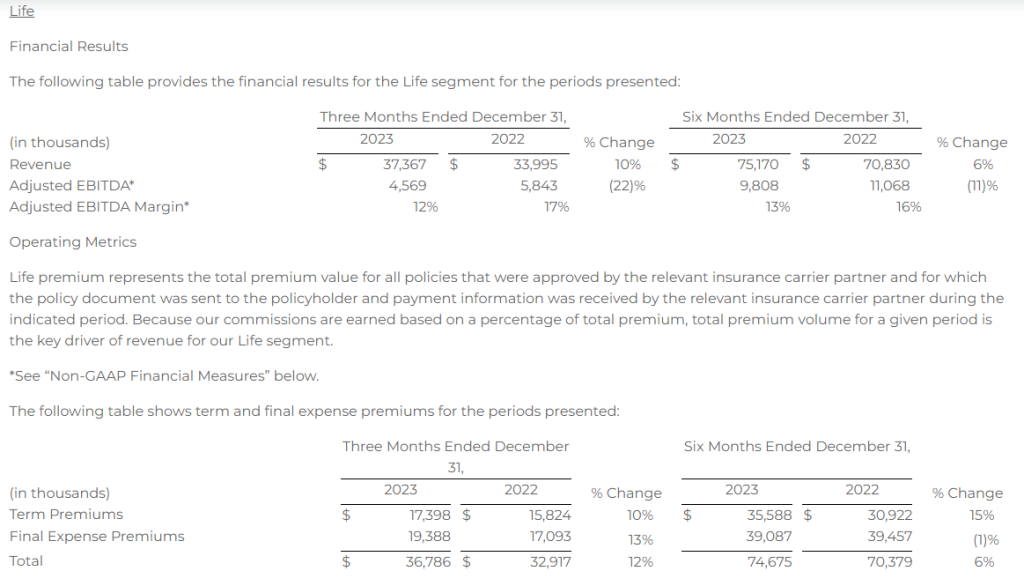
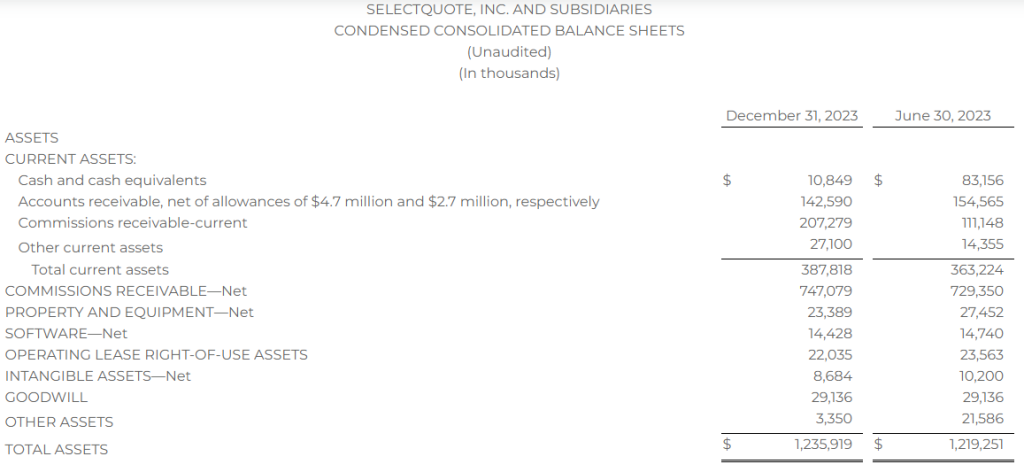
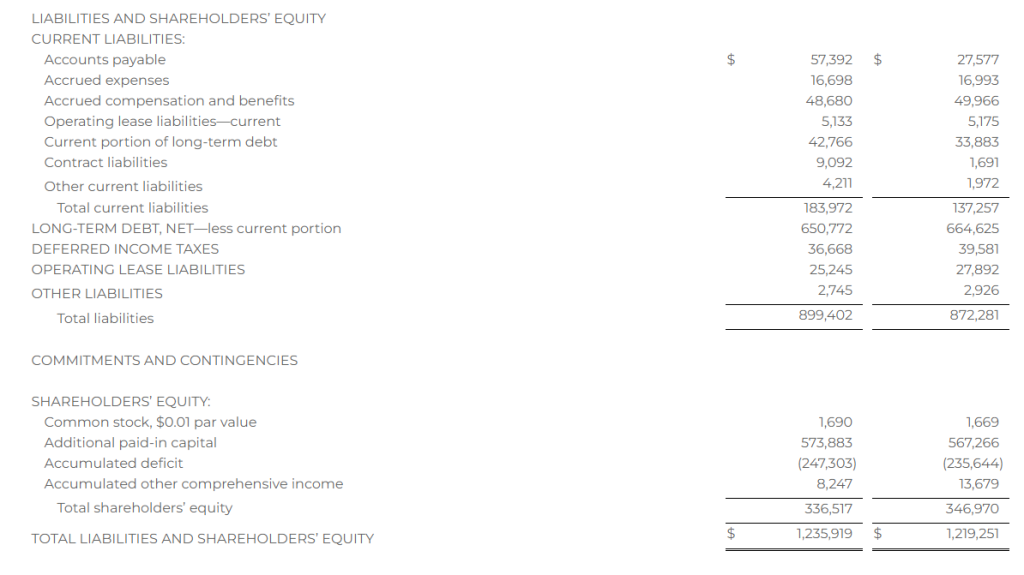
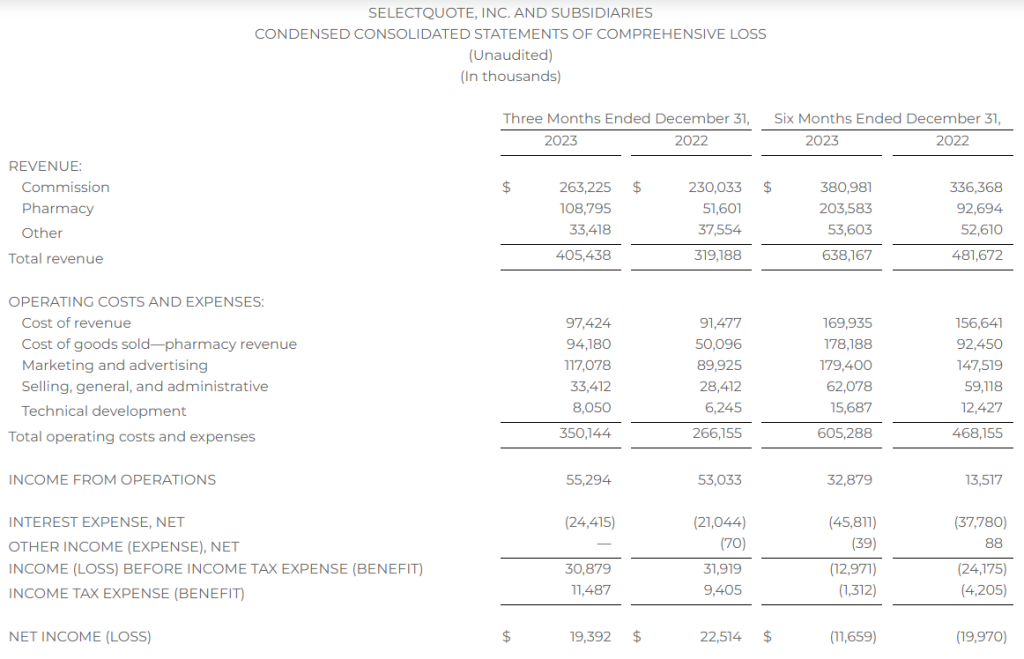
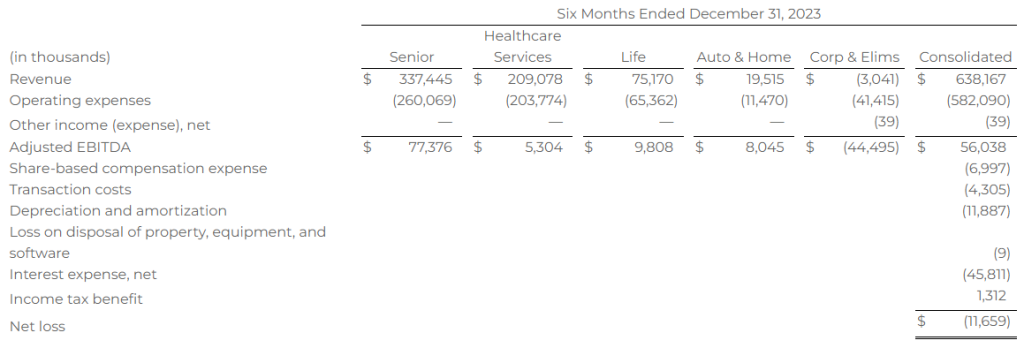
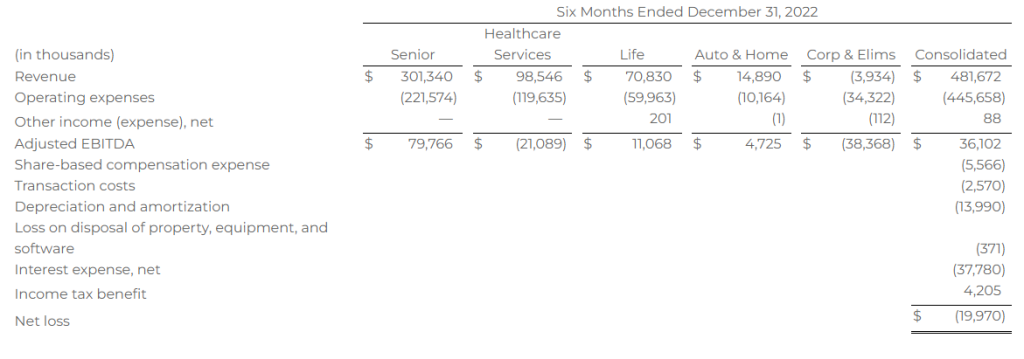
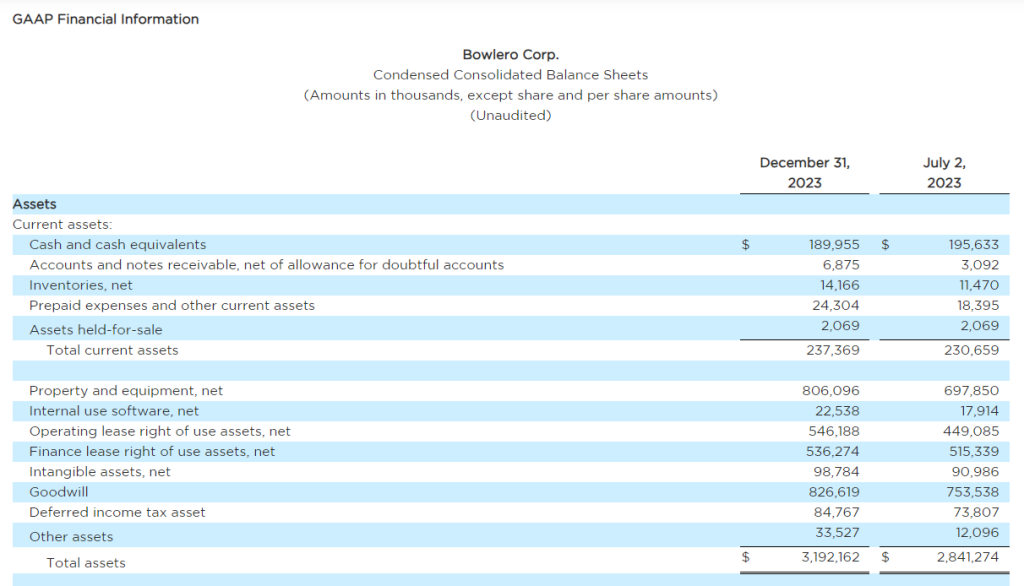
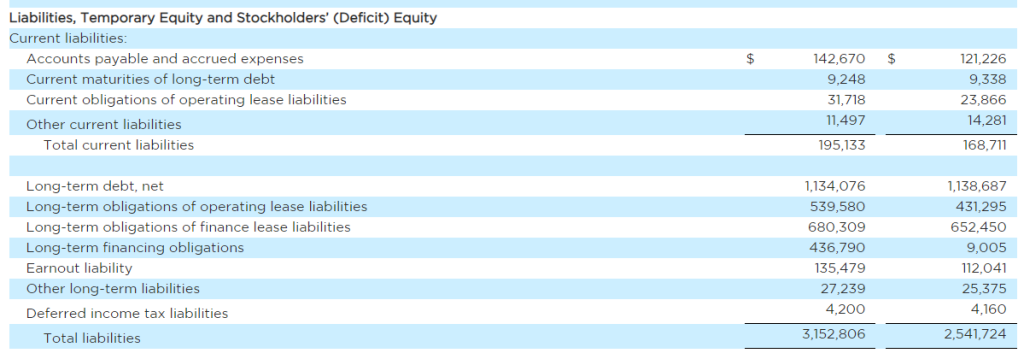
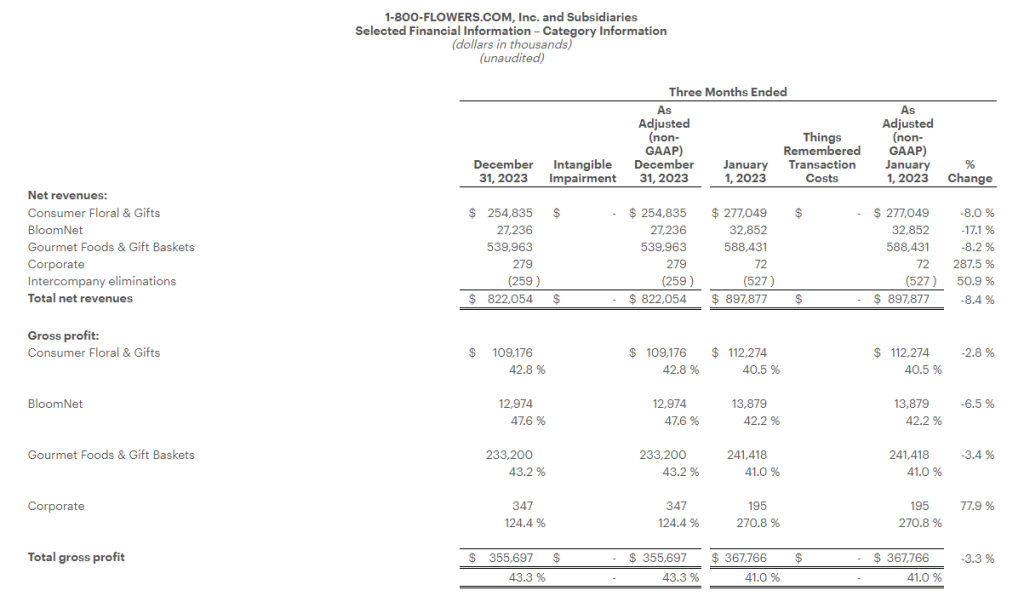
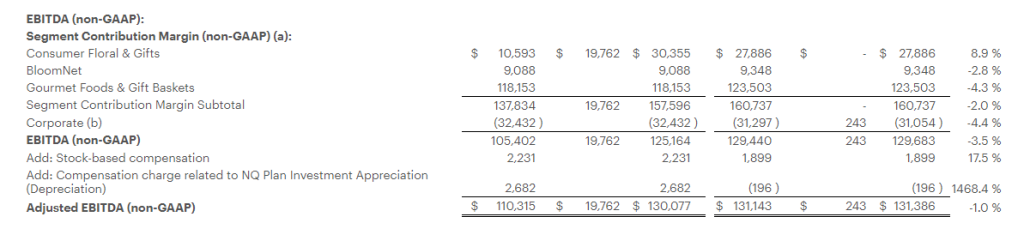
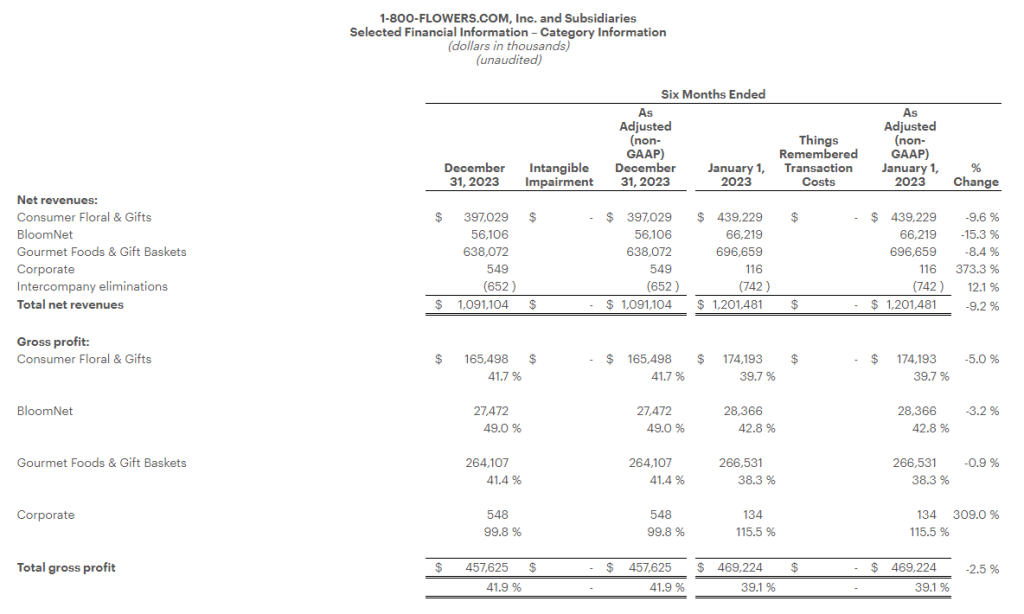
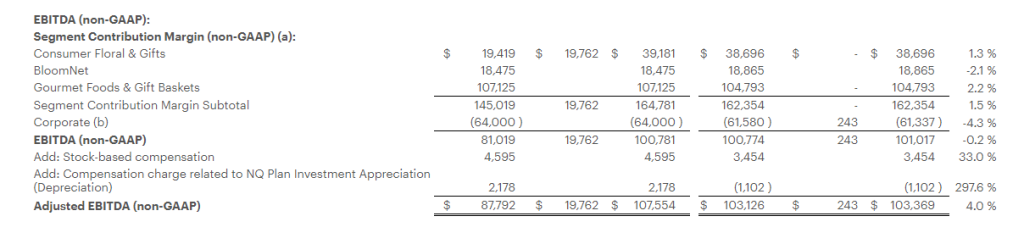
Research News and Market Data on MAIA
February 07, 2024 8:01am EST
- THIO treatment leads to profound activation of innate and adaptive anti-tumor responses
- THIO depletes cancer initiating cells (CICs) and thus diminishes tumor initiation and metastasis-forming potential in various in vivo models
- THIO previously awarded orphan drug designation (ODD) by FDA for small cell lung cancer (SCLC) treatment
CHICAGO–(BUSINESS WIRE)– MAIA Biotechnology, Inc., (NYSE American: MAIA) (“MAIA”, the “Company”), a clinical-stage biopharmaceutical company developing targeted immunotherapies for cancer, today announced the publication of extensive work describing preclinical studies for lead candidate THIO in small cell lung cancer (SCLC) in the peer-reviewed scientific journal Nature Communications. The reported findings from the research, conducted in collaboration with the University of Texas Southwestern (UTSW) scientists, led by corresponding author Dr. Esra Akbay, demonstrate the immune-enhancing, metastasis-reducing effects of MAIA’s telomere-targeting agent THIO (6TdG) in several well-characterized in vitro and in vivo models of SCLC.
“This publication highlights a rather unique dual mechanism of action for THIO as a first-in-clinic telomere-targeted anticancer agent for potential treatment of SCLC,” said Sergei M. Gryaznov, PhD., MAIA’s Chief Scientific Officer. “In addition to the direct and potent cancer cell depletion activity, the observed specific interferons stimulation, immune responses-enhancement, and metastasis-reducing effects of THIO provide solid scientific foundation for further advancement of this compound in clinical development.”
A prominent characteristic of lung cancer small cells is their reliance on telomerase activity, a key enzyme essential for the continuous proliferation of SCLC. While 85-90% of all human cancers are telomerase positive, SCLCs are nearly all telomerase positive1, suggesting that telomerase targeting may be an effective strategy in the treatment of SCLC.
Key findings in the published paper include:
- Human and mouse SCLC lines are sensitive to THIO (6TdG) treatment in vitro and in vivo
- THIO decreases cancer initiating cells and diminishes tumor initiation potential in vitro and in vivo
- Low doses of THIO are effective in treating metastatic mouse SCLC tumors
- THIO activates type-I interferon pathway through cGAS-STING signaling
- THIO is highly effective in combination with ionizing radiation treatment regiments
“With few, if any, effective treatments for small cell lung cancer, there is a widespread need for innovative therapeutic strategies. The positive outcomes reported in our publication show THIO’s potential as a new therapeutic approach,” said Vlad Vitoc, M.D., MAIA’s Chairman and Chief Executive Officer. “THIO already holds Orphan Drug Designation for SCLC, underscoring the FDA’s recognition of THIO’s potential to improve outcomes for this highly lethal disease. With the positive preclinical and clinical data we have obtained to date for THIO, we have entered the Phase 2 planning stage for a clinical trial of THIO in SCLC along with two other cancers.”
Orphan Products Development grants orphan designation status to drugs and biologics that are intended for the treatment, diagnosis or prevention of rare diseases, or conditions that affect fewer than 200,000 people in the U.S. Orphan Drug Designation provides certain benefits, including financial incentives, to support clinical development and the potential for up to seven years of market exclusivity for the drug for the designated orphan indication in the U.S. if the drug is ultimately approved for its designated indication.
About the Publication
Nature Communications, volume 15, article number: 672 (2024), “A telomere-targeting drug depletes cancer initiating cells and promotes anti-tumor immunity in small cell lung cancer,” published 22 January 2024. Co-author disclosures included in manuscript.
About Small Cell Lung Cancer
Small cell lung cancer (SCLC) accounts for 13% of lung cancers. As the deadliest of all lung cancers, SCLC is one of the leading causes of cancer-related mortality in United States with 30,000 deaths annually. It is less common than non-small cell lung cancer (NSCLC), but is more aggressive and rapidly spreads (metastasizes) throughout the body.
About THIO
THIO (6-thio-dG or 6-thio-2’-deoxyguanosine) is a first-in-class investigational telomere-targeting agent currently in clinical development to evaluate its activity in Non-Small Cell Lung Cancer (NSCLC). Telomeres, along with the enzyme telomerase, play a fundamental role in the survival of cancer cells and their resistance to current therapies. The modified nucleoside 6-thio-2’-deoxyguanosine (THIO) induces telomerase-dependent telomeric DNA modification, DNA damage responses, and selective cancer cell death. THIO-damaged telomeric fragments accumulate in cytosolic micronuclei activating both innate (cGAS/STING) and adaptive (T-cell) immune responses. The sequential treatment with THIO followed by PD-(L)1 inhibitors resulted in profound and persistent tumor regression in advanced, in vivo cancer models by induction of cancer type–specific immune memory. THIO is presently developed as a second or later line of treatment for NSCLC for patients that have progressed beyond the standard-of-care regimen of existing checkpoint inhibitors.
About MAIA Biotechnology, Inc.
MAIA is a targeted therapy, immuno-oncology company focused on the development and commercialization of potential first-in-class drugs with novel mechanisms of action that are intended to meaningfully improve and extend the lives of people with cancer. Our lead program is THIO, a potential first-in-class cancer telomere targeting agent in clinical development for the treatment of NSCLC patients with telomerase-positive cancer cells. For more information, please visit www.maiabiotech.com.
Forward Looking Statements
MAIA cautions that all statements, other than statements of historical facts contained in this press release, are forward-looking statements. Forward-looking statements are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels or activity, performance or achievements to be materially different from those anticipated by such statements. The use of words such as “may,” “might,” “will,” “should,” “could,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify forward looking statements. However, the absence of these words does not mean that statements are not forward-looking. For example, all statements we make regarding (i) the initiation, timing, cost, progress and results of our preclinical and clinical studies and our research and development programs, (ii) our ability to advance product candidates into, and successfully complete, clinical studies, (iii) the timing or likelihood of regulatory filings and approvals, (iv) our ability to develop, manufacture and commercialize our product candidates and to improve the manufacturing process, (v) the rate and degree of market acceptance of our product candidates, (vi) the size and growth potential of the markets for our product candidates and our ability to serve those markets, and (vii) our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates, are forward looking. All forward-looking statements are based on current estimates, assumptions and expectations by our management that, although we believe to be reasonable, are inherently uncertain. Any forward-looking statement expressing an expectation or belief as to future events is expressed in good faith and believed to be reasonable at the time such forward-looking statement is made. However, these statements are not guarantees of future events and are subject to risks and uncertainties and other factors beyond our control that may cause actual results to differ materially from those expressed in any forward-looking statement. Any forward-looking statement speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. In this release, unless the context requires otherwise, “MAIA,” “Company,” “we,” “our,” and “us” refers to MAIA Biotechnology, Inc. and its subsidiaries.
_________________________________
1 Hiyama, K. et al. Telomerase activity in small-cell and non-small-cell lung cancers. J Natl Cancer Inst 87, 895-902, doi:10.1093/jnci/87.12.895 (1995).
View source version on businesswire.com: https://www.businesswire.com/news/home/20240207835567/en/
Investor Relations Contact
+1 (872) 270-3518
ir@maiabiotech.com
Source: MAIA Biotechnology, Inc.
Released February 7, 2024