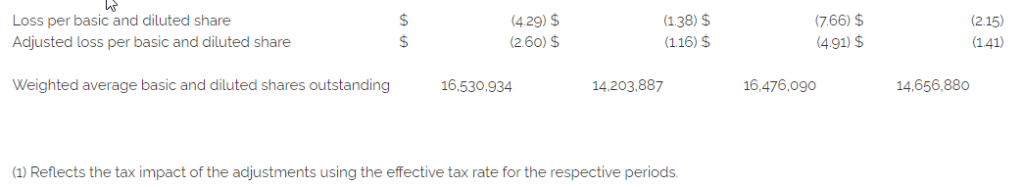Research News and Market Data on FAT
FEBRUARY 22, 2023
Conference call and webcast today at 5:00 p.m. ET
LOS ANGELES, Feb. 22, 2023 (GLOBE NEWSWIRE) — FAT (Fresh. Authentic. Tasty.) Brands Inc. (NASDAQ: FAT) (“FAT Brands” or the “Company”) today reported fourth quarter and full year 2022 financial results for the fiscal year ended December 25, 2022.
Andy Wiederhorn, President and CEO of FAT Brands, commented, “The fourth quarter marked yet another strong performance for FAT Brands, as evidenced by our robust unit development and profitable revenue growth. After a very active acquisition strategy in 2021, I am particularly pleased with the momentum of our organic growth strategy during 2022.”
“With over 140 store openings during 2022, we achieved a new milestone for FAT Brands, including 44 that opened in the fourth quarter. We plan to continue this robust unit growth with between 150 and 175 units slated to open in 2023. We are seeing strong new franchisee activity as well as continued demand from existing franchise partners to develop other brands within our portfolio, which is very encouraging as we look beyond our current unit development pipeline of over 1,000 locations representing 60% EBITDA growth over the next several years.”
“We are extremely impressed with how our 2021 acquisitions have seamlessly fit into our portfolio and the demand we are experiencing for them from our franchisee base. In addition to our organic growth momentum, we will lean into the expansion of our high-growth brands, particularly our sports lodge category, and continue to expand our factory business.”
“We also continue to work on reducing our cost of capital and are pursuing strategies to significantly reduce our leverage ratio over the next 24 to 36 months.”
Fiscal Fourth Quarter 2022 Highlights
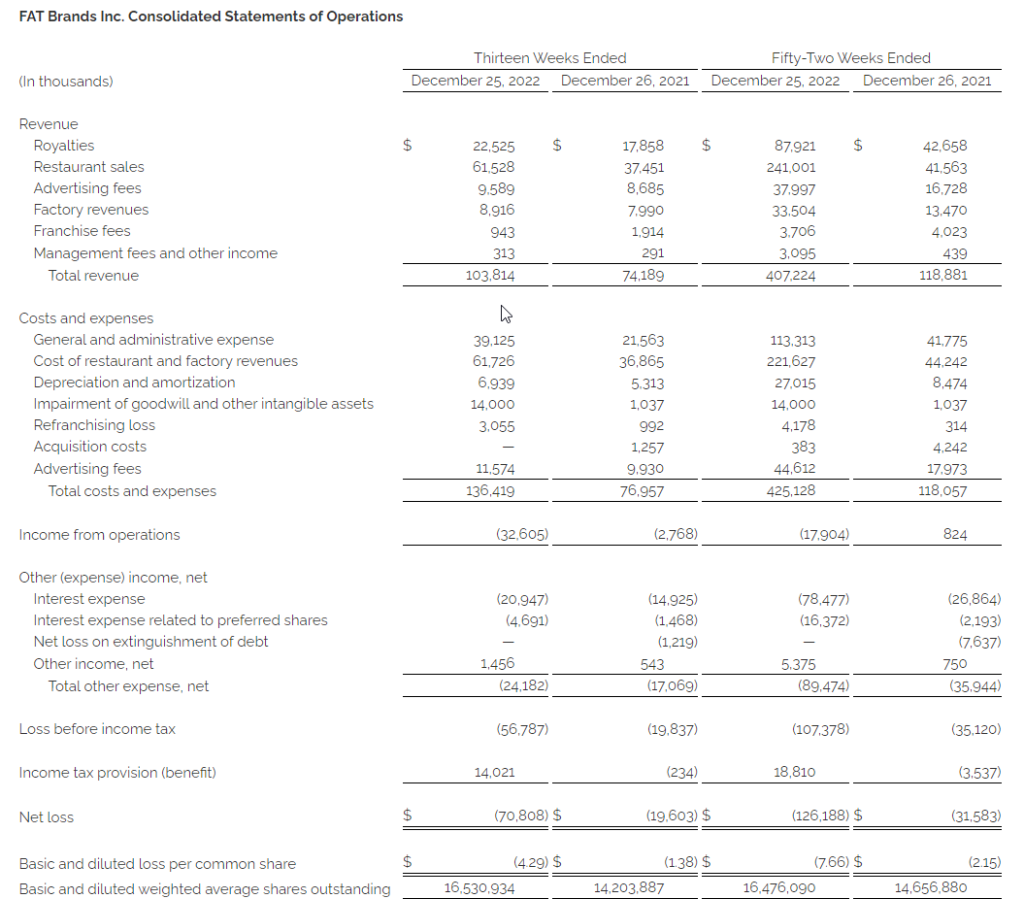
- Total revenue improved 39.9% to $103.8 million compared to $74.2 million in the fourth quarter of 2021
- System-wide sales growth of 22.1% in the fourth quarter of 2022 compared to the prior year quarter
- Year-to-date system-wide same-store sales growth of 2.7% in the fourth quarter of 2022 compared to the prior year
- 44 new store openings during the fourth quarter of 2022 and over 140 openings during the year
- Net loss of $70.8 million, or $4.29 per diluted share, compared to $19.6 million, or $1.38 per diluted share, in the fourth quarter of 2021
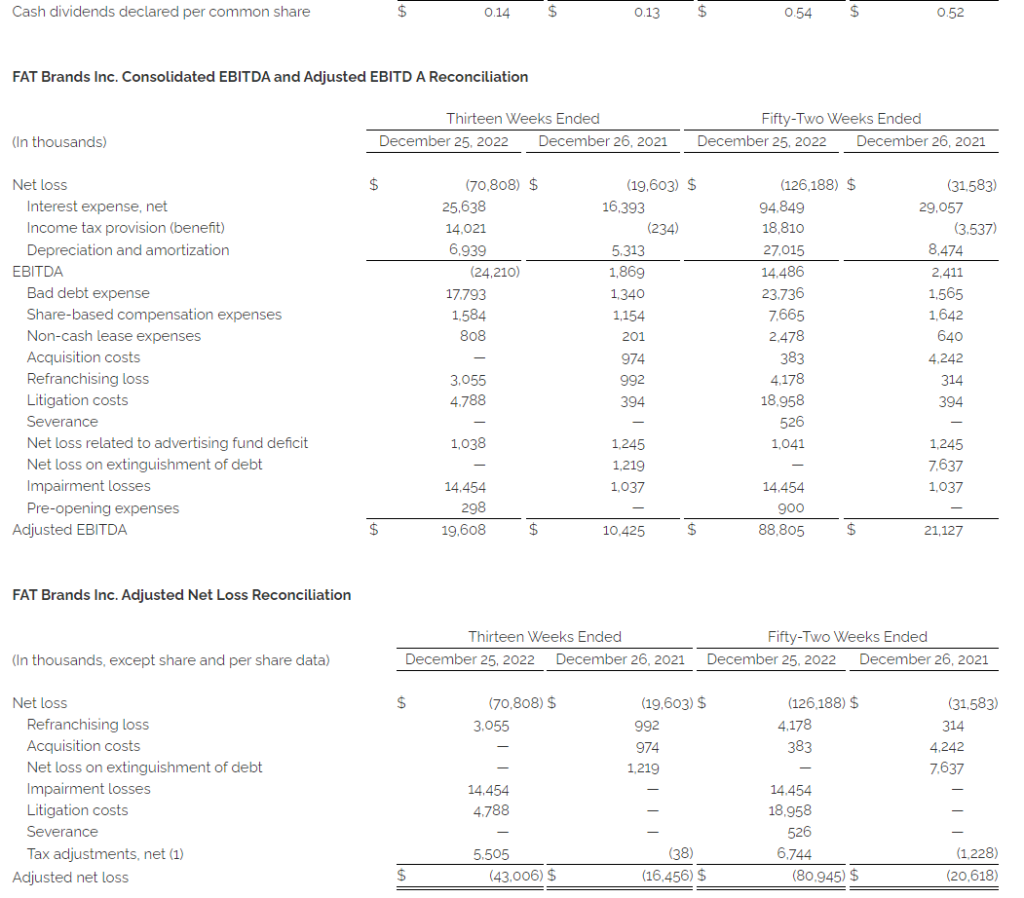
- Adjusted EBITDA(1) of $19.6 million compared to $10.4 million in the fourth quarter of 2021
- Adjusted net loss(1) of $43.0 million, or $2.60 per diluted share, compared to $16.5 million, or $1.16 per diluted share, in the fourth quarter of 2021
Fiscal Year 2022 Highlights
- Total revenue increased 242.5% to $407.2 million compared to $118.9 million in 2021
- System-wide sales growth of 108.0% compared to 2021
- Year-to-date system-wide same-store sales growth of 6.0% in 2022 compared to 2021
- Over 140 new store openings during 2022
- Net loss of $126.2 million, or $7.66 per diluted share, compared to $31.6 million, or $2.15 per diluted share, in 2021
- Adjusted EBITDA(1) of $88.8 million compared to $21.1 million 2021
- Adjusted net loss(1) of $80.9 million, or $4.91 per diluted share, compared to $20.6 million, or $1.41 per diluted share, in 2021
(1) EBITDA, Adjusted EBITDA and adjusted net loss are non-GAAP measures defined below, under “Non-GAAP Measures”. Reconciliation of GAAP net loss to EBITDA, adjusted EBITDA and adjusted net loss are included in the accompanying financial tables.
Summary of Fourth Quarter 2022 Financial Results
Total revenue increased $29.6 million, or 39.9%, in the fourth quarter of 2022, to $103.8 million compared to $74.2 million in the same period of 2021. The increase reflects revenue from the acquisition of Twin Peaks in October 2021, the acquisitions of Fazoli’s and Native Grill & Wings in December 2021 (collectively, the “2021 Acquisitions”) and the continuing recovery from the negative effects of the COVID-19 pandemic on royalties from restaurant sales.
Costs and expenses increased $59.5 million, or 77.3%, in the fourth quarter of 2022 to $136.4 million compared to $77.0 million in the same period in the prior year, primarily due to the 2021 Acquisitions.
General and administrative expense increased $17.6 million, or 81.4%, in the fourth quarter of 2022 compared to the same period in the prior year, primarily due to the 2021 Acquisitions, increased compensation costs, professional fees related to pending litigation and government investigations, and travel, reflecting the significant expansion of the organization.
Cost of restaurant and factory revenues totaled $61.7 million in the fourth quarter of 2022 and was related to the operations of the company-owned restaurant locations and our dough factory associated with the 2021 Acquisitions.
Depreciation and amortization increased $1.6 million, or 30.6% in the fourth quarter of 2022 compared to the same period in the prior year, primarily due to depreciation of company-owned restaurant property and equipment and amortizing intangible assets related to the 2021 Acquisitions.
Refranchising losses in the fourth quarter of 2022 were $3.1 million and were comprised of restaurant costs and expenses, net of food sales. Refranchising losses in the fourth quarter of 2021 were $1.0 million and were comprised of $2.1 million restaurant operating costs, net of food sales, partially offset by $1.1 million in net gains related to refranchised restaurants.
Advertising expenses increased $1.6 million in the fourth quarter of 2022 compared to the prior year period. These expenses vary in relation to advertising revenues and reflect advertising expenses related to the 2021 Acquisitions and the increase in customer activity as the recovery from COVID continues.
Total other expense, net for the fourth quarters of 2022 and 2021 was $24.2 million and $17.1 million, respectively, primarily comprised of net interest expense of $25.6 million and $16.4 million, respectively.
Adjusted net loss was $43.0 million, or $2.60 per diluted share, in the fourth quarter of 2022 compared to $16.5 million, or $1.16 per diluted share, in the fourth quarter of 2021.
Key Financial Definitions
New store openings – The number of new store openings reflects the number of stores opened during a particular reporting period. The total number of new stores per reporting period and the timing of stores openings has, and will continue to have, an impact on our results.
Same-store sales growth – Same-store sales growth reflects the change in year-over-year sales for the comparable store base, which we define as the number of stores open and in the FAT Brands system for at least one full fiscal year. For stores that were temporarily closed, sales in the current and prior period are adjusted accordingly. Given our focused marketing efforts and public excitement surrounding each opening, new stores often experience an initial start-up period with considerably higher than average sales volumes, which subsequently decrease to stabilized levels after three to six months. Additionally, when we acquire a brand, it may take several months to integrate fully each location of said brand into the FAT Brands platform. Thus, we do not include stores in the comparable base until they have been open and in the FAT Brands system for at least one full fiscal year. For 2022, the comparable store base does not include concepts acquired during the fourth quarter of 2021.
System-wide sales growth – System wide sales growth reflects the percentage change in sales in any given fiscal period compared to the prior fiscal period for all stores in that brand only when the brand is owned by FAT Brands. Because of acquisitions, new store openings and store closures, the stores open throughout both fiscal periods being compared may be different from period to period.
Conference Call and Webcast
FAT Brands will host a conference call and webcast to discuss its fiscal fourth quarter 2022 financial results today at 5:00 PM ET. Hosting the conference call and webcast will be Andy Wiederhorn, President and Chief Executive Officer, and Ken Kuick, Chief Financial Officer.
The conference call can be accessed live over the phone by dialing 1-877-704-4453 from the U.S. or 1-201-389-0920 internationally. A replay will be available after the call until Wednesday, March 1, 2023, and can be accessed by dialing 1-844-512-2921 from the U.S. or 1-412-317-6671 internationally. The passcode is 13735781. The webcast will be available at www.fatbrands.com under the “Investors” section and will be archived on the site shortly after the call has concluded.
About FAT (Fresh. Authentic. Tasty.) Brands
FAT Brands (NASDAQ: FAT) is a leading global franchising company that strategically acquires, markets, and develops fast casual, quick-service, casual dining, and polished casual dining concepts around the world. The Company currently owns 17 restaurant brands: Round Table Pizza, Fatburger, Marble Slab Creamery, Johnny Rockets, Fazoli’s, Twin Peaks, Great American Cookies, Hot Dog on a Stick, Buffalo’s Cafe & Express, Hurricane Grill & Wings, Pretzelmaker, Elevation Burger, Native Grill & Wings, Yalla Mediterranean and Ponderosa and Bonanza Steakhouses and franchises and owns approximately 2,300 units worldwide. For more information, please visit www.fatbrands.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements relating to the future financial and operating results of the Company, estimates of future EBITDA, the timing and performance of new store openings, future reductions in cost of capital and leverage ratio, our ability to conduct future accretive acquisitions, our pipeline of new store locations, and the recovery of our business from the COVID-19 pandemic. Forward-looking statements generally use words such as “expect,” “foresee,” “anticipate,” “believe,” “project,” “should,” “estimate,” “will,” “plans,” “forecast,” and similar expressions, and reflect our expectations concerning the future. Forward-looking statements are subject to significant business, economic and competitive risks, uncertainties and contingencies, many of which are difficult to predict and beyond our control, which could cause our actual results to differ materially from the results expressed or implied in such forward-looking statements. We refer you to the documents that we file from time to time with the Securities and Exchange Commission, such as our reports on Form 10-K, Form 10-Q and Form 8-K, for a discussion of these and other risks and uncertainties that could cause our actual results to differ materially from our current expectations and from the forward-looking statements contained in this press release. We undertake no obligation to update any forward-looking statements to reflect events or circumstances occurring after the date of this press release.
Non-GAAP Measures (Unaudited)
This press release includes the non-GAAP financial measures of EBITDA, adjusted EBITDA and adjusted net loss.
EBITDA is defined as earnings before interest, taxes, and depreciation and amortization. We use the term EBITDA, as opposed to income from operations, as it is widely used by analysts, investors, and other interested parties to evaluate companies in our industry. We believe that EBITDA is an appropriate measure of operating performance because it eliminates the impact of expenses that do not relate to business performance. EBITDA is not a measure of our financial performance or liquidity that is determined in accordance with generally accepted accounting principles (“GAAP”), and should not be considered as an alternative to net income (loss) as a measure of financial performance or cash flows from operations as measures of liquidity, or any other performance measure derived in accordance with GAAP.
Adjusted EBITDA is defined as EBITDA (as defined above), excluding expenses related to acquisitions, refranchising gain or losses, impairment charges, and certain non-recurring or non-cash items that the Company does not believe directly reflect its core operations and may not be indicative of the Company’s recurring business operations.
Adjusted net loss is a supplemental measure of financial performance that is not required by or presented in accordance with GAAP. Adjusted net loss is defined as net loss plus the impact of adjustments and the tax effects of such adjustments. Adjusted net loss is presented because we believe it helps convey supplemental information to investors regarding our performance, excluding the impact of special items that affect the comparability of results in past quarters to expected results in future quarters. Adjusted net loss as presented may not be comparable to other similarly titled measures of other companies, and our presentation of adjusted net loss should not be construed as an inference that our future results will be unaffected by excluded or unusual items. Our management uses this non-GAAP financial measure to analyze changes in our underlying business from quarter to quarter based on comparable financial results.
Reconciliations of net loss presented in accordance with GAAP to EBITDA, adjusted EBITDA and adjusted net loss are set forth in the tables below.
Investor Relations:
ICR
Michelle Michalski
ir-fatbrands@icrinc.com
646-277-1224
Media Relations:
Erin Mandzik
emandzik@fatbrands.com
860-212-6509