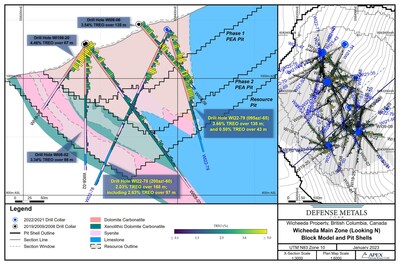
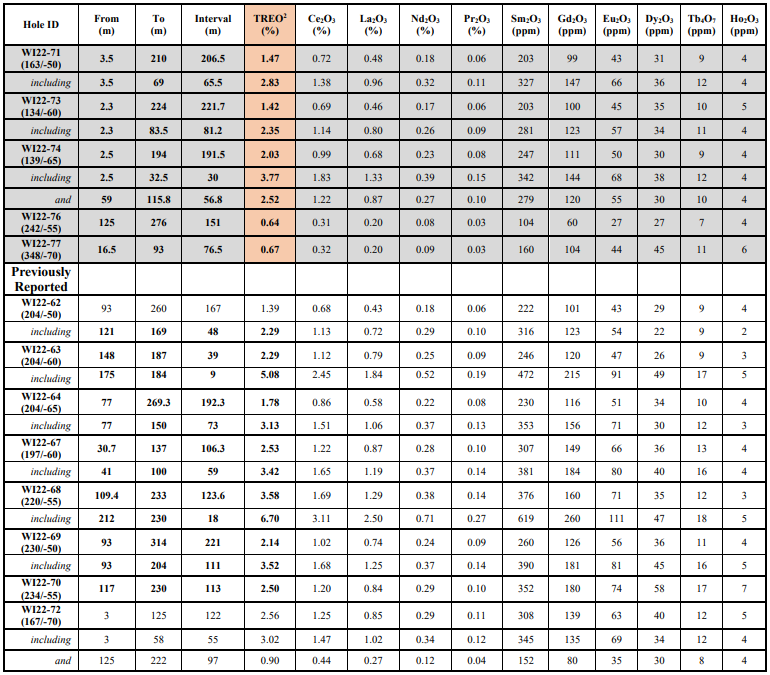
Research News and Market Data on BXRX
January 24, 2023 8:00am EST
MALVERN, Pa., Jan. 24, 2023 (GLOBE NEWSWIRE) — Baudax Bio, Inc. (NASDAQ:BXRX) a pharmaceutical company focused on innovative products for hospital and related settings, today announced the successful outcome of its first interim analysis in a Phase II trial of BX1000 for neuromuscular blockade (NMB) in patients undergoing elective surgery.
“We are encouraged by the results of the first interim analysis of the BX1000 Phase II surgery trial,” said Gerri Henwood, Baudax Bio’s President and Chief Executive Officer. “We believe the use of BX1000, combined with our reversal agent, BX3000, could make for precise control of timing under neuromuscular paralysis for surgical patients, which could result in time and cost savings for patients and hospitals alike. We look forward to completing enrollment in the study in Q1 and sharing topline results for the study in April 2023.”
This randomized, double-blind, active-controlled clinical trial comparing three different doses of BX1000 to a standard dose of rocuronium is planned to enroll a total of 80 adult patients undergoing elective surgery utilizing total intravenous anesthesia. The primary efficacy endpoint is the proportion of patients meeting criteria for Good or Excellent intubating conditions using a standardized scale. Additionally, the trial is evaluating the safety and tolerability profile of BX1000 and rocuronium in this patient population.
This pre-planned interim analysis evaluated the intubating conditions for each patient after administration of study drug in a blinded fashion. In the 20-patient cohort, 5 patients per group received one of the study medications. All 20 patients were observed to have met the criteria for Good or Excellent intubating conditions at 60 seconds. Nineteen of the subjects were successfully intubated following the assessment at 60 seconds, and the one remaining subject following the assessment at 90 seconds. Study treatments were generally well tolerated with no occurrence of severe or serious adverse events.
This blinded interim analysis did not result in the decision to drop any of the four study groups nor any decision to adjust planned study enrollment numbers.
About Baudax Bio’s Neuromuscular Blocking Agents (NMBs)
Baudax Bio holds exclusive global rights to two novel NMBs, BX1000, an intermediate duration, clinical stage blocking agent, and BX2000, an ultra-short duration, clinical stage blocking agent, as well as a proprietary chemical reversal agent, BX3000, undergoing nonclinical studies intended to support an IND filing in 2023. BX3000 is a specific reversal agent that rapidly reverses BX1000 and BX2000. All three agents are licensed from Cornell University. We believe these agents allow for a very rapid induction of neuromuscular blockade for surgical settings, followed by a rapid reversal of the neuromuscular blockade. These novel agents have the potential to meaningfully reduce time to onset of blocking and of reversal of blockade, reducing time in operating rooms or post-acute care settings, resulting in potential clinical and cost advantages, as well as time-related valuable cost savings for hospitals and ambulatory surgical centers.
About Baudax Bio
Baudax Bio is a pharmaceutical company focused on innovative products for hospital and related settings. The Company has a pipeline of innovative pharmaceutical assets including two clinical-stage, novel neuromuscular blocking (NMBs) agents, one in a Phase II study and an additional unique NMB in a dose escalation Phase I study, as well as a proprietary chemical reversal agent specific to these NMBs. Baudax Bio has received approval for and marketed ANJESO®, the first and only 24-hour, intravenous (IV) COX-2 preferential non-opioid, non-steroidal anti-inflammatory (NSAID) for the management of moderate to severe pain. For more information, please visit www.baudaxbio.com.
Forward-Looking Statements
This press release contains forward-looking statements that involve risks and uncertainties. Such forward-looking statements reflect Baudax Bio’s expectations about its future performance and opportunities that involve substantial risks and uncertainties. When used herein, the words “anticipate,” “believe,” “estimate,” “may,” “upcoming,” “plan,” “target,” “goal,” “intend” and “expect” and similar expressions, as they relate to Baudax Bio or its management, are intended to identify such forward-looking statements. These forward-looking statements, which include statements relating to the development of each of BX1000 and BX3000, are based on information available to Baudax Bio as of the date of this press release and are subject to a number of risks, uncertainties, and other factors that could cause actual results to differ materially from those expressed in, or implied by, these forward-looking statements. These risks and uncertainties include, among other things, risks related to market, economic and other conditions, the ongoing economic and social consequences of the COVID-19 pandemic, Baudax Bio’s ability to advance its current product candidate pipeline through pre-clinical studies and clinical trials, Baudax Bio’s ability to raise future financing for continued development of its product candidates such as BX1000, BX2000 and BX3000, Baudax Bio’s ability to pay its debt and satisfy conditions necessary to access future tranches of debt, Baudax Bio’s ability to comply with the financial and other covenants under its credit facility, Baudax Bio’s ability to manage costs and execute on its operational and budget plans, Baudax Bio’s ability to achieve its financial goals; Baudax Bio’s ability to comply with all listing requirements of the Nasdaq Capital Market; and Baudax Bio’s ability to obtain, maintain and successfully enforce adequate patent and other intellectual property protection. These forward-looking statements should be considered together with the risks and uncertainties that may affect Baudax Bio’s business and future results included in Baudax Bio’s filings with the Securities and Exchange Commission at www.sec.gov. These forward-looking statements are based on information currently available to Baudax Bio, and Baudax Bio assumes no obligation to update any forward-looking statements except as required by applicable law.
CONTACT:
Investor Relations Contact:
Argot Partners
Sam Martin / Kaela Ilami
(212) 600-1902
baudaxbio@argotpartners.com
Media Contact:
Argot Partners
David Rosen
(212) 600-1902
david.rosen@argotpartners.com
Source: Baudax Bio, Inc.
Released January 24, 2023