
Research News and Market Data on CNDT
February 12, 2025
Key Q4 and Full Year 2024 Highlights
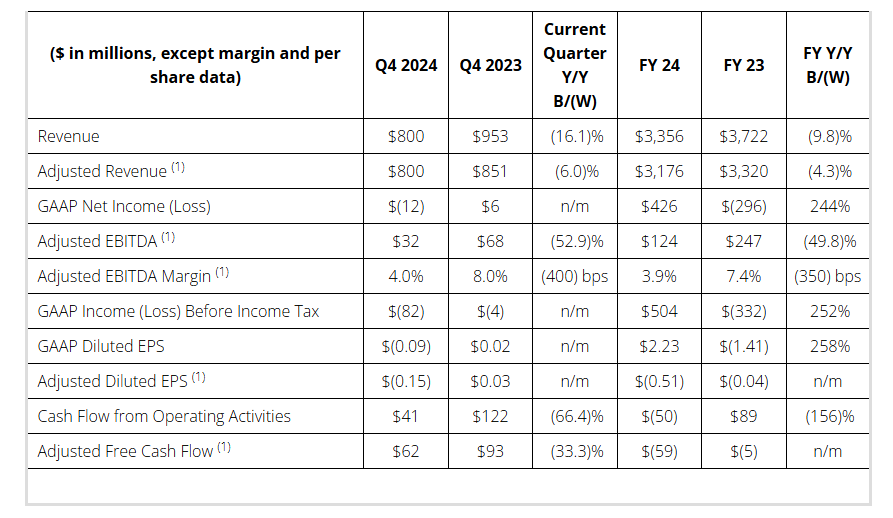
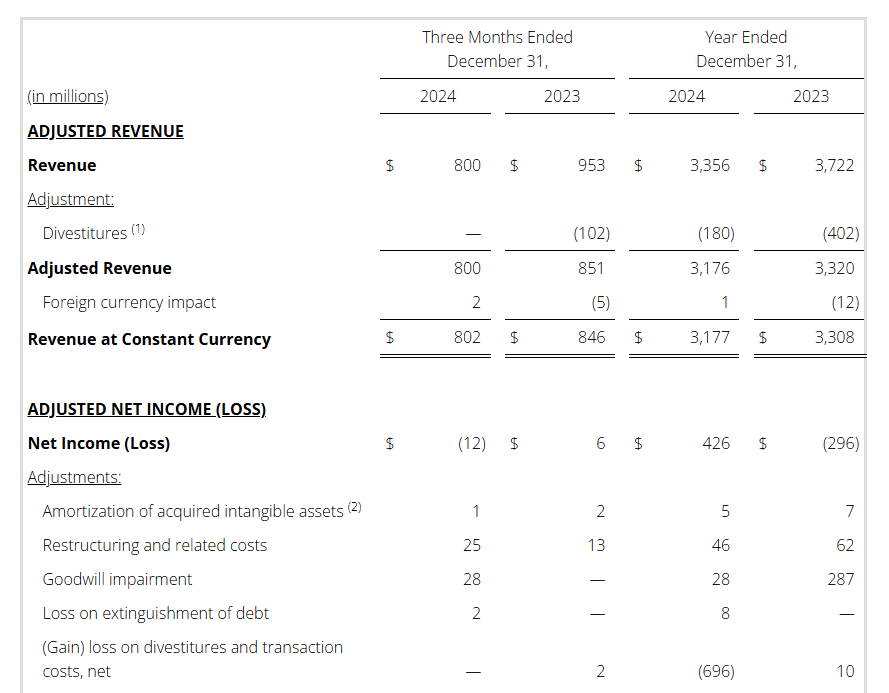
- Revenue: Q4 $800M / FY $3,356M
- Adj. Revenue (1) : Q4 $800M / FY $3,176M
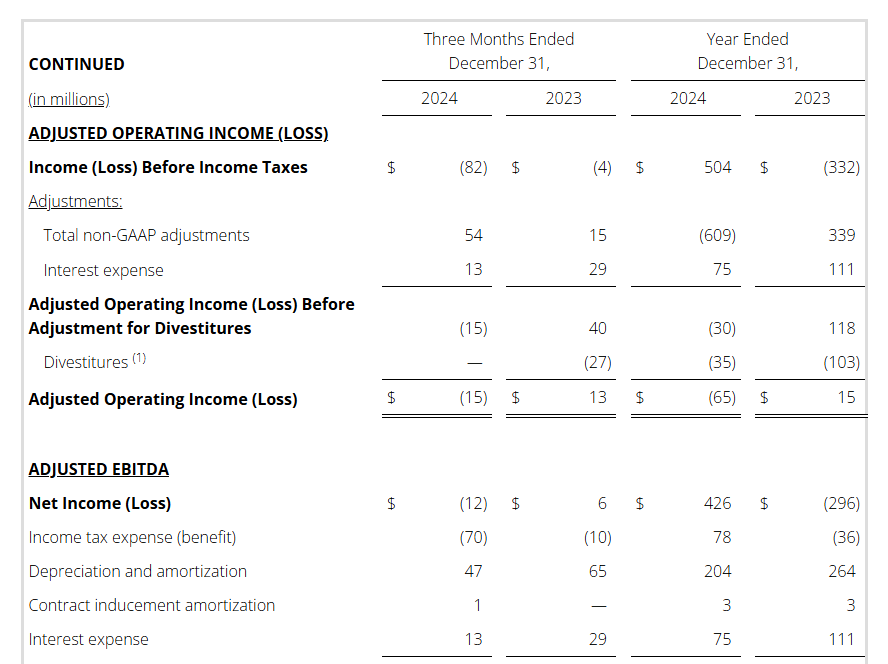
- Pre-tax Income (Loss): Q4 $(82)M / FY $504M
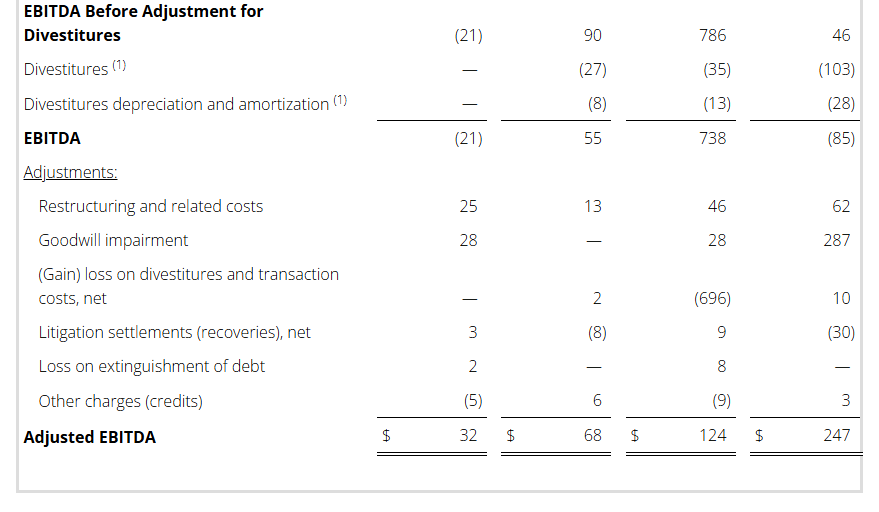
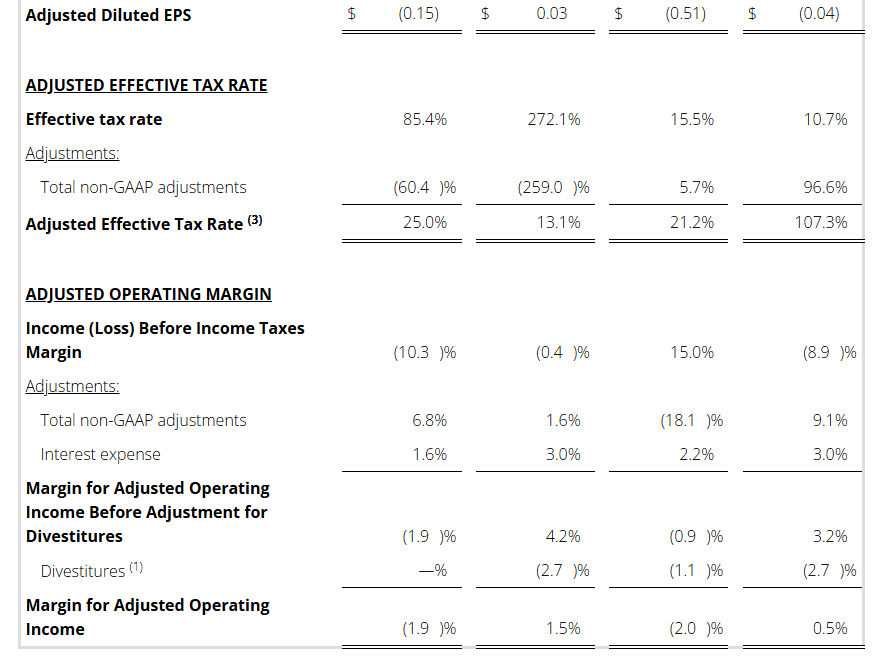
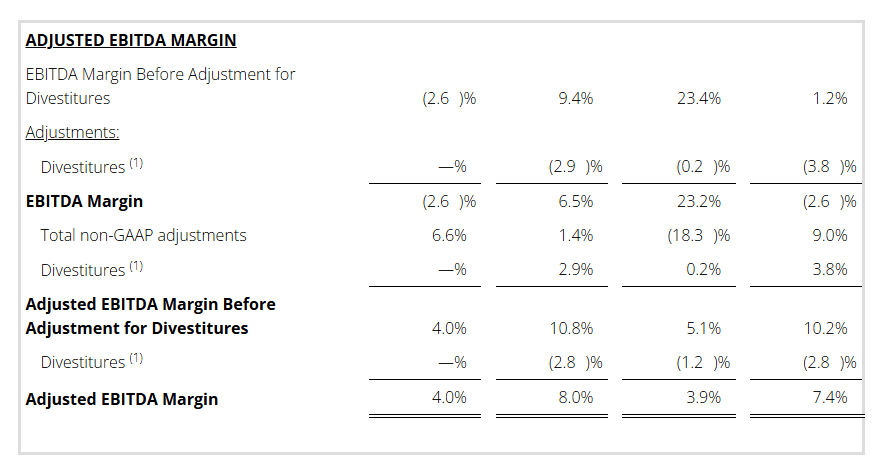
- Adj. EBITDA Margin (1) : Q4 4.0% / FY 3.9%
- New Business Signings ACV (2) : Q4 $137M / FY $485M
- Net ARR Activity Metric (2) (TTM): $92M
FLORHAM PARK, N.J., Feb. 12, 2025 — Conduent Incorporated (Nasdaq: CNDT), a global technology-led business process solutions and services company, today announced its fourth quarter and full year 2024 financial results.
Cliff Skelton, Conduent President and Chief Executive Officer stated, “2024 proved to be broadly in line with what we planned for. It was a year we said would be characterized by a continued shift to growth, with a focus on new leadership, a rationalized portfolio, improved industry recognition, and improved client retention. It was all of that and more, enhanced by divestitures with solid multiples and a 50% reduction in debt compared to year-end 2023.”
“From a numbers perspective, while timing drove a slightly weaker top line finish to the year, it was offset by an EBITDA margin on the high end of expectations. Quarterly Adjusted Revenue improved sequentially for the past three quarters and Adjusted EBITDA also increased over the past three quarters.”
“We remain bullish on achieving expectations in 2025. We continue to see opportunities for a further rationalized portfolio and remain focused on delivering outstanding service to our valued client base.”
Key Financial Q4 & Full Year 2024 Results
Performance Commentary
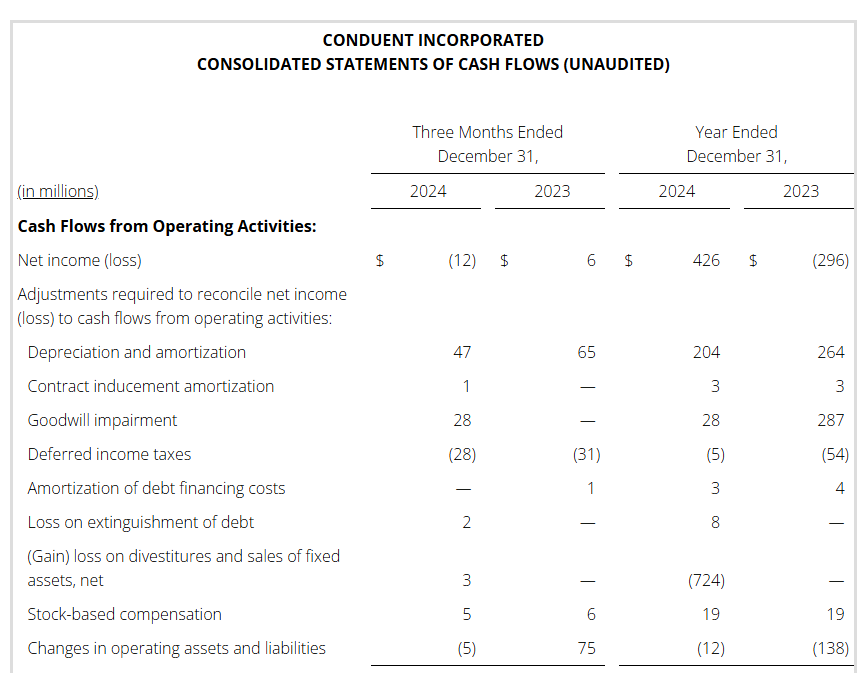
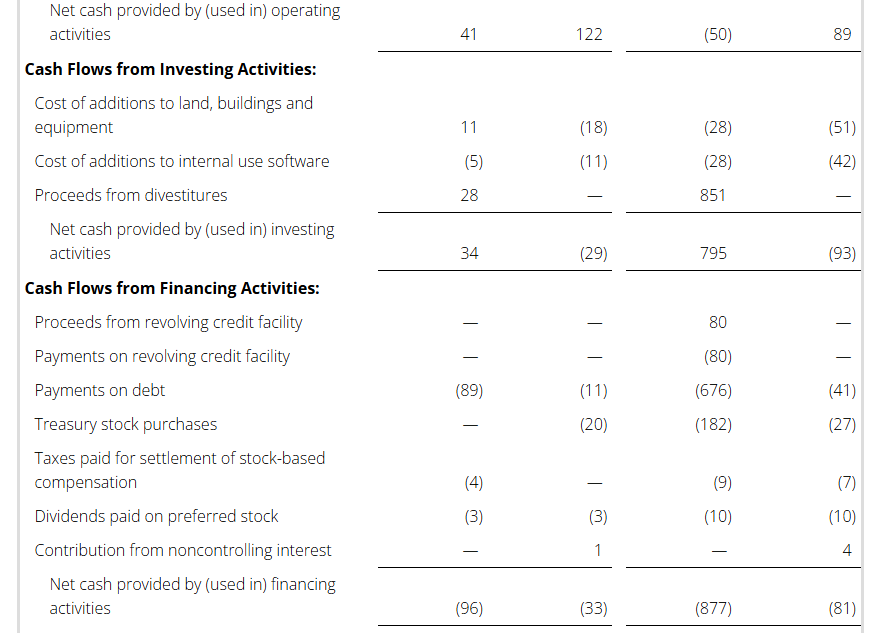
During 2024, the Company completed three divestitures as part of its portfolio rationalization strategy. The transfer of the BenefitWallet portfolio was completed during the second quarter of 2024 for a total purchase price of $425 million. During the second quarter of 2024, the company also completed the sale of the Curbside Management and Public Safety businesses with a purchase price of $230 million, $50 million of which is deferred to the first half of 2025. During the third quarter of 2024, the company completed the sale of the Casualty Claims Solutions Business and received $224 million of cash consideration.
Also, during 2024, the Company used a portion of the proceeds from the divested businesses to voluntarily prepay all of the principal of the Term Loan B and $137 million of the Term Loan A.
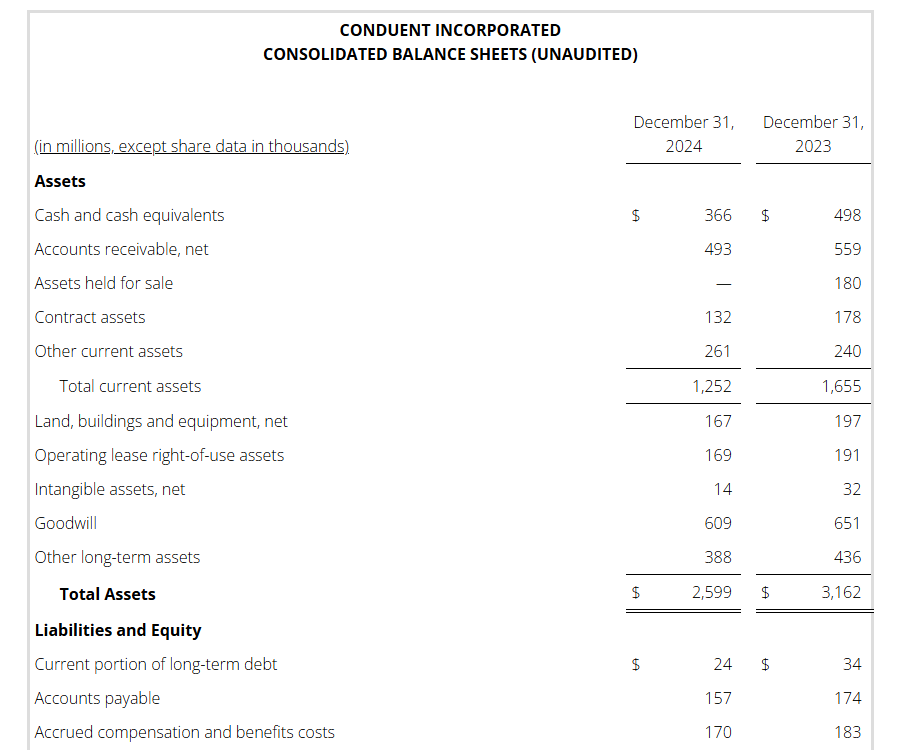
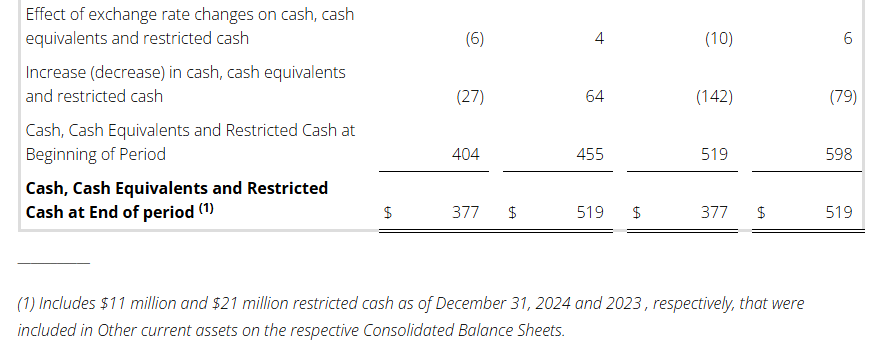
Conduent’s liquidity position remains strong with long-dated debt maturities and a modest net leverage ratio.
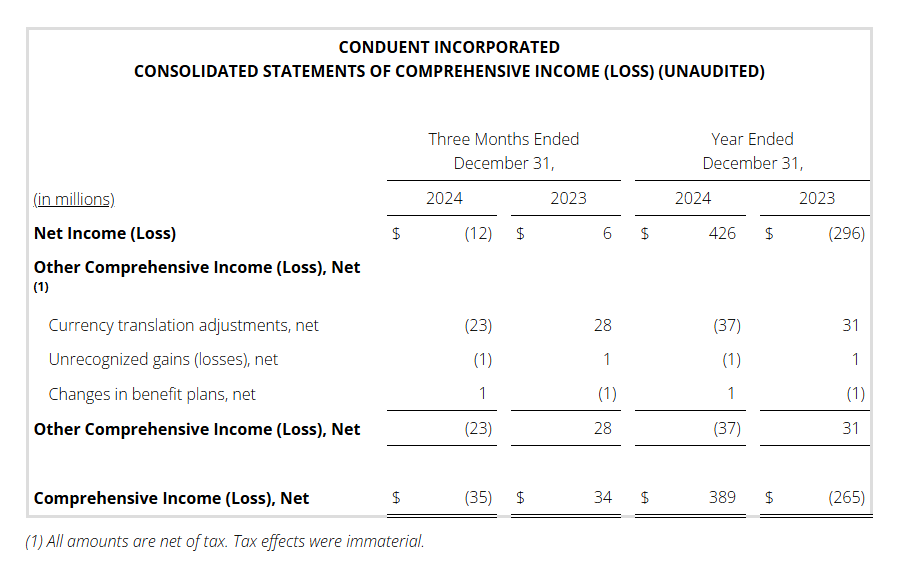
Full year 2024 pre-tax income (loss) was $504 million versus $(332) million in the prior year. This increase is primarily driven by the gain on the sale of the three divested businesses noted above, as well as a goodwill impairment in the prior year.
During 2024 the Company completed its previously approved $75 million share repurchase program and bought back a total of 52 million shares of common stock, including approximately 38 million shares purchased from Carl Icahn and affiliates.
Additional Q4 & Full Year 2024 Performance Highlights
Conduent achieved several milestones in technology-led solutions, operational excellence and culture, including:
- Announced several implementations and advanced solutions in Transportation including expanded 3D fare gates, open payment digital wallet fare collection and all-electronic express lane tolling for clients in the US and Europe;
- Implemented several digital payment solutions for several states that combat fraud and disburse payments to those in need;
- Integrated AI-driven solutions by TALON and Jellyvision’s ALEX with Conduent’s Life@Work Connect Experience Platform to enhance employee benefits decisions;
- Collaborated with Microsoft on an initiative across the Conduent portfolio to drive innovation using Microsoft Azure OpenAI Services;
- Earned Leader Recognition from:
- Information Services Group (ISG) as a U.S. and Europe “Leader” in its 2024 Contact Center – Customer Experience Services Provider Lens™ report; and
- NelsonHall’s NEAT Report for Healthcare Payer Operational Transformation; CX Services Transformation – Cost Optimization Focus; and Multi-Process HR Transformation Services for Large Enterprises.
- Earned Recognition for Industry Leadership and Culture:
- “GovTech Top 100 Company” for the third consecutive year;
- Newsweek Top 100 Most Loved Workplaces for third consecutive year;
- “Best Place to Work for Disability Inclusion” (Disability Equality Index); and
- Forbes’ list of America’s Best Employers for Diversity for the fourth consecutive year.
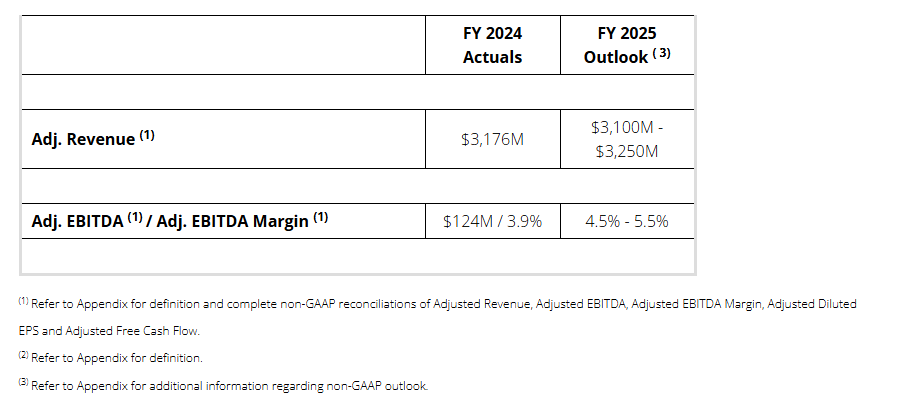
FY 2025 Outlook
Conference Call
Management will present the results during a conference call and webcast on February 12, 2025 at 9:00 a.m. ET.
The call will be available by live audio webcast along with the news release and online presentation slides at https://investor.conduent.com/.
The conference call will also be available by calling 877-407-4019 toll-free. If requested, the conference ID for this call is 13750544.
The international dial-in is 1-201-689-8337. The international conference ID is also 13750544.
A recording of the conference call will be available by calling 1-877-660-6853 three hours after the conference call concludes. The replay ID is 13750544.
The telephone recording will be available until February 26, 2025.
About Conduent
Conduent delivers digital business solutions and services spanning the commercial, government and transportation spectrum – creating valuable outcomes for its clients and the millions of people who count on them. The company leverages cloud computing, artificial intelligence, machine learning, automation and advanced analytics to deliver mission-critical solutions. Through a dedicated global team of approximately 56,000 associates, process expertise and advanced technologies, Conduent’s solutions and services digitally transform its clients’ operations to enhance customer experiences, improve performance, increase efficiencies and reduce costs. Conduent adds momentum to its clients’ missions in many ways including disbursing approximately $85 billion in government payments annually, enabling approximately 2.3 billion customer service interactions annually, empowering millions of employees through HR services every year and processing over 13 million tolling transactions every day. Learn more at www.conduent.com.
Non-GAAP Financial Measures
We have reported our financial results in accordance with accounting principles generally accepted in the U.S. (U.S. GAAP). In addition, we have discussed our financial results using non-GAAP measures. We believe these non-GAAP measures allow investors to better understand the trends in our business and to better understand and compare our results. Accordingly, we believe it is necessary to adjust several reported amounts, determined in accordance with U.S. GAAP, to exclude the effects of certain items as well as their related tax effects. Management believes that these non-GAAP financial measures provide an additional means of analyzing the results of the current period against the corresponding prior period. However, these non-GAAP financial measures should be viewed in addition to, and not as a substitute for, our reported results prepared in accordance with U.S. GAAP. Our non-GAAP financial measures are not meant to be considered in isolation or as a substitute for comparable U.S. GAAP measures and should be read only in conjunction with our Consolidated Financial Statements prepared in accordance with U.S. GAAP. Our management regularly uses our non-GAAP financial measures internally to understand, manage and evaluate our business and make operating decisions. Providing such non-GAAP financial measures to investors allows for a further level of transparency as to how management reviews and evaluates our business results and trends. These non-GAAP measures are among the primary factors management uses in planning for and forecasting future periods. Compensation of our executives is based in part on the performance of our business based on certain of these non-GAAP measures. Refer to the “Non-GAAP Financial Measures” section attached to this release for a discussion of these non-GAAP measures and their reconciliation to the reported U.S. GAAP measures.
Forward-Looking Statements
This press release, any exhibits or attachments to this release, and other public statements we make may contain “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995. The words “anticipate,” “believe,” “estimate,” “expect,” “expectations,” “in front of us,” “plan,” “intend,” “will,” “aim,” “should,” “could,” “forecast,” “target,” “may,” “continue to,” “looking to continue,” “endeavor,” “if,” “growing,” “projected,” “potential,” “likely,” “see,” “ahead,” “further,” “going forward,” “on the horizon,” “as we progress,” “going to,” “path from here forward,” “think,” “path to deliver,” “from here,” and similar expressions (including the negative and plural forms of such words and phrases), as they relate to us, are intended to identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. All statements other than statements of historical fact included in this press release or any attachment to this press release are forward-looking statements, including, but not limited to, statements regarding our financial results, condition and outlook; changes in our operating results; general market and economic conditions; and our projected financial performance, including all statements made under the section captioned “FY 2025 Outlook” within this release. These statements reflect our current views with respect to future events and are subject to certain risks, uncertainties and assumptions, many of which are outside of our control, that could cause actual results to differ materially from those expected or implied by such forward-looking statements contained in this press release, any exhibits to this press release and other public statements we make.
Important factors and uncertainties that could cause our actual results to differ materially from those in our forward-looking statements include, but are not limited to: government appropriations and termination rights contained in our government contracts, the competitiveness of the markets in which we operate and our ability to renew commercial and government contracts, including contracts awarded through competitive bidding processes; our ability to recover capital and other investments in connection with our contracts; our reliance on third-party providers; risk and impact of geopolitical events and increasing geopolitical tensions (such as the war in the Ukraine and conflict in the Middle East), macroeconomic conditions, natural disasters and other factors in a particular country or region on our workforce, customers and vendors; our ability to deliver on our contractual obligations properly and on time; changes in interest in outsourced business process services; claims of infringement of third-party intellectual property rights; our ability to estimate the scope of work or the costs of performance in our contracts; the loss of key senior management and our ability to attract and retain necessary technical personnel and qualified subcontractors; our failure to develop new service offerings and protect our intellectual property rights; our ability to modernize our information technology infrastructure and consolidate data centers; expectations relating to environmental, social and governance considerations; utilization of our stock repurchase program; risks related to our use of artificial intelligence; the failure to comply with laws relating to individually identifiable information and personal health information; the failure to comply with laws relating to processing certain financial transactions, including payment card transactions and debit or credit card transactions; breaches of our information systems or security systems or any service interruptions; our ability to comply with data security standards; developments in various contingent liabilities that are not reflected on our balance sheet, including those arising as a result of being involved in a variety of claims, lawsuits, investigations and proceedings; risks related to recently completed divestitures including the (i) transfer of the Company’s BenefitWallet’s health savings account, medical savings account and flexible spending account portfolio, (ii) the sale of the Company’s Curbside Management and Public Safety Solutions businesses and (iii) the sale of the Company’s Casualty Claims Solutions business, including but not limited to the Company’s ability to realize the benefits anticipated from such transactions, unexpected costs, liabilities or delays in connection with such transactions, and the significant transaction costs associated with such transactions; risk and impact of potential goodwill and other asset impairments; our significant indebtedness and the terms of such indebtedness; our failure to obtain or maintain a satisfactory credit rating and financial performance; our ability to obtain adequate pricing for our services and to improve our cost structure; our ability to collect our receivables, including those for unbilled services; a decline in revenues from, or a loss of, or a reduction in business from or failure of significant clients; fluctuations in our non-recurring revenue; increases in the cost of voice and data services or significant interruptions in such services; our ability to receive dividends or other payments from our subsidiaries; and other factors that are set forth in the “Risk Factors” section, the “Legal Proceedings” section, the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section and other sections in our 2024 Annual Report on Form 10-K, as well as in our Quarterly Reports on Form 10-Q and Current Reports on Form 8-K filed with or furnished to the Securities and Exchange Commission. Any forward-looking statements made by us in this release speak only as of the date on which they are made. We are under no obligation to, and expressly disclaim any obligation to, update or alter our forward-looking statements, whether because of new information, subsequent events or otherwise, except as required by law.
###
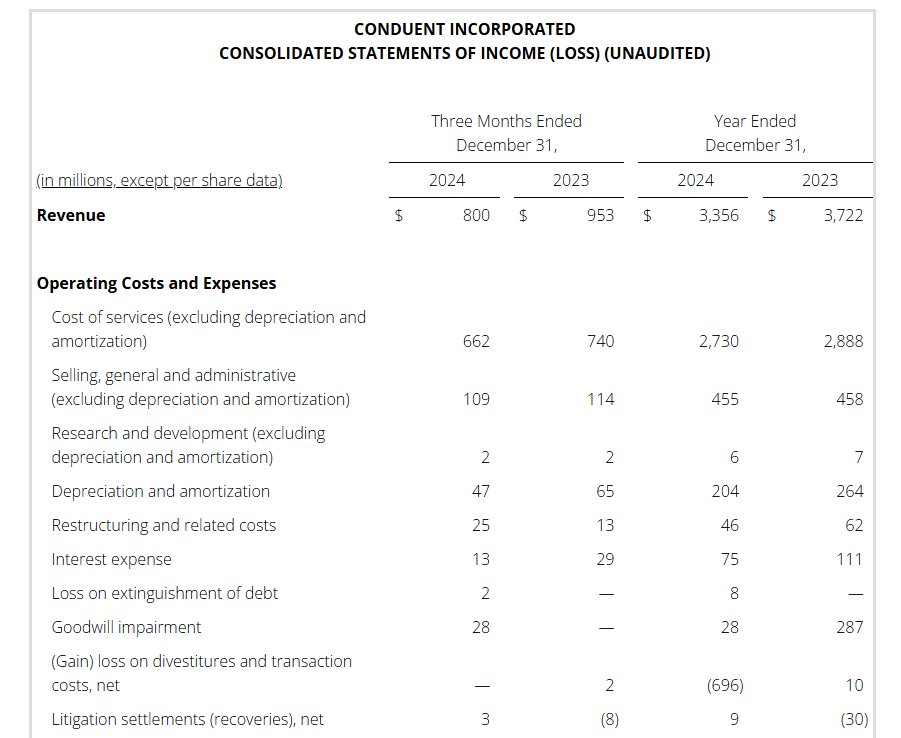


Appendix
Definitions
Net ARR Activity Metric (TTM)
Projected Annual Recurring Revenue (ARR) for contracts signed in the prior 12 months, less the annualized impact of any client losses, contractual volume and price changes, and other known impacts for which the company was notified in that same time period, which could positively or negatively impact results. The metric annualizes the net impact to revenue. Timing of revenue impact varies and may not be realized within the forward 12-month timeframe. The metric is for indicative purposes only. This metric excludes non-recurring revenue signings. This metric is not indicative of any specific 12 month timeframe.
New Business Annual Contract Value (ACV): (New Business TCV / contract term) multiplied by 12.
New Business Total Contract Value (TCV): Estimated total future revenues from contracts signed during the period related to new logo, new service line or expansion with existing customers.
TTM: Trailing twelve months.
PBT: Profit before tax.
Non-GAAP Financial Measures
We have reported our financial results in accordance with accounting principles generally accepted in the U.S. (U.S. GAAP). In addition, we have discussed our financial results using non-GAAP measures.
We believe these non-GAAP measures allow investors to better understand the trends in our business and to better understand and compare our results. Accordingly, we believe it is necessary to adjust several reported amounts, determined in accordance with U.S. GAAP, to exclude the effects of certain items as well as their related tax effects. Management believes that these non-GAAP financial measures provide an additional means of analyzing the results of the current period against the corresponding prior period. However, these non-GAAP financial measures should be viewed in addition to, and not as a substitute for, the Company’s reported results prepared in accordance with U.S. GAAP. Our non-GAAP financial measures are not meant to be considered in isolation or as a substitute for comparable U.S. GAAP measures and should be read only in conjunction with our Consolidated Financial Statements prepared in accordance with U.S. GAAP. Our management regularly uses our non-GAAP financial measures internally to understand, manage and evaluate our business and make operating decisions, and providing such non-GAAP financial measures to investors allows for a further level of transparency as to how management reviews and evaluates our business results and trends. These non-GAAP measures are among the primary factors management uses in planning for and forecasting future periods. Compensation of our executives is based in part on the performance of our business based on certain of these non-GAAP measures.
Management cautions that amounts presented in accordance with Conduent’s definition of non-GAAP financial measures may not be comparable to similar measures disclosed by other companies because not all companies calculate non-GAAP measures in the same manner.
A reconciliation of the following non-GAAP financial measures to the most directly comparable financial measures calculated and presented in accordance with U.S. GAAP are provided below.
These reconciliations also include the income tax effects for our non-GAAP performance measures in total, to the extent applicable. The income tax effects are calculated under the same accounting principles as applied to our reported pre-tax performance measures under Accounting Standards Codification 740, which employs an annual effective tax rate method. The noted income tax effect for our non-GAAP performance measures is effectively the difference in income taxes for reported and adjusted pre-tax income calculated under the annual effective tax rate method. The tax effect of the non-GAAP adjustments was calculated based upon evaluation of the statutory tax treatment and the applicable statutory tax rate in the jurisdictions in which such charges were incurred.
Adjusted Revenue, Adjusted Profit Before Tax, Adjusted Net Income (Loss), Adjusted Diluted Earnings per Share, Adjusted Weighted Average Common Shares Outstanding, and Adjusted Effective Tax Rate
We make adjustments to Revenue, Net Income (Loss) before Income Taxes for the following items, as applicable, to the particular financial measure, for the purpose of calculating Adjusted Revenue, Adjusted Profit Before Tax, Adjusted Net Income (Loss), Adjusted Diluted Earnings per Share, Adjusted Weighted Average Common Shares Outstanding, and Adjusted Effective Tax Rate:
- Amortization of acquired intangible assets. The amortization of acquired intangible assets is driven by acquisition activity, which can vary in size, nature and timing as compared to other companies within our industry and from period to period.
- Restructuring and related costs. Restructuring and related costs include restructuring and asset impairment charges as well as costs associated with our strategic transformation program.
- Goodwill impairment. This represents goodwill impairment charges arising from annual or interim goodwill testing.
- (Gain) loss on divestitures and transaction costs, net. Represents (gain) loss on divested businesses and transaction costs.
- Litigation settlements (recoveries), net represents settlements or recoveries for various matters subject to litigation.
- Loss on extinguishment of debt. This represents write-off related debt issuance costs related to prepayments of debt.
- Other charges (credits). This includes Other (income) expenses, net on the Consolidated Statements of Income (loss) and other adjustments.
- Divestitures. Revenue and Adjusted EBITDA of divested businesses are excluded.
The Company provides adjusted net income and adjusted EPS financial measures to assist our investors in evaluating our ongoing operating performance for the current reporting period and, where provided, over different reporting periods, by adjusting for certain items which may be recurring or non-recurring and which in our view do not necessarily reflect ongoing performance. We also internally use these measures to assess our operating performance, both absolutely and in comparison to other companies, and in evaluating or making selected compensation decisions.
Management believes that the adjusted effective tax rate, provided as supplemental information, facilitates a comparison by investors of our actual effective tax rate with an adjusted effective tax rate which reflects the impact of the items which are excluded in providing adjusted net income and certain other identified items, and may provide added insight into our underlying business results and how effective tax rates impact our ongoing business.
Adjusted Revenue, Adjusted Operating Income and Adjusted Operating Margin
We make adjustments to Revenue, Costs and Expenses and Operating Margin for the following items, as applicable, for the purpose of calculating Adjusted Revenue, Adjusted Operating Income and Adjusted Operating Margin:
- Amortization of acquired intangible assets.
- Restructuring and related costs.
- Interest expense. Interest expense includes interest on long-term debt and amortization of debt issuance costs.
- Goodwill impairment.
- Loss on extinguishment of debt.
- (Gain) loss on divestitures and transaction costs, net.
- Litigation settlements (recoveries), net.
- Other charges (credits).
- Divestitures.
We provide our investors with adjusted revenue, adjusted operating income and adjusted operating margin information, as supplemental information, because we believe it offers added insight, by itself and for comparability between periods, by adjusting for certain non-cash items as well as certain other identified items which we do not believe are indicative of our ongoing business, and may also provide added insight on trends in our ongoing business.
Adjusted EBITDA and EBITDA Margin
We use Adjusted EBITDA and Adjusted EBITDA Margin as an additional way of assessing certain aspects of our operations that, when viewed with the U.S. GAAP results and the accompanying reconciliations to corresponding U.S. GAAP financial measures, provide a more complete understanding of our on-going business. Adjusted EBITDA represents income (loss) before interest, income taxes, depreciation and amortization and contract inducement amortization adjusted for the following items. Adjusted EBITDA Margin is Adjusted EBITDA divided by revenue or adjusted revenue, as applicable.
- Restructuring and related costs.
- Goodwill impairment.
- Loss on extinguishment of debt.
- (Gain) loss on divestitures and transaction costs, net.
- Litigation settlements (recoveries), net.
- Other charges (credits).
- Divestitures.
Adjusted EBITDA is not intended to represent cash flows from operations, operating income (loss) or net income (loss) as defined by U.S. GAAP as indicators of operating performance.
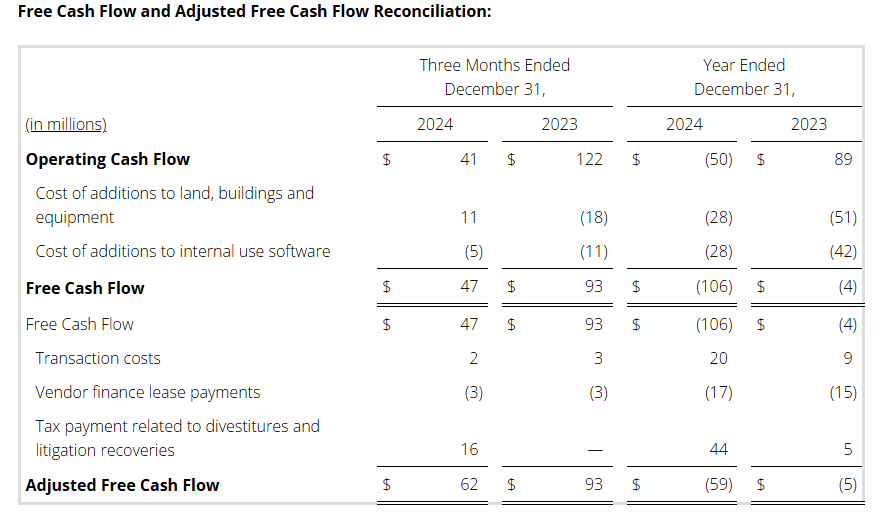
Free Cash Flow
Free Cash Flow is defined as cash flows from operating activities as reported on the consolidated statement of cash flows, less cost of additions to land, buildings and equipment, cost of additions to internal use software, and proceeds from sales of land, buildings and equipment, as applicable. We use the non-GAAP measure of Free Cash Flow as a criterion of liquidity. We use Free Cash Flow as a measure of liquidity to determine amounts we can reinvest in our core businesses, such as amounts available to make acquisitions and invest in land, buildings and equipment and internal use software, after required payments on debt. In order to provide a meaningful basis for comparison, we are providing information with respect to our Free Cash Flow reconciled to cash flow provided by operating activities, which we believe to be the most directly comparable measure under U.S. GAAP.
Adjusted Free Cash Flow
Adjusted Free Cash Flow is defined as Free Cash Flow from above plus adjustments for litigation insurance recoveries, transaction costs, taxes paid on gains from divestitures and litigation recoveries, proceeds from failed sale-leaseback transactions and certain other identified adjustments, as applicable. We use Adjusted Free Cash Flow, in addition to Free Cash Flow, to provide supplemental information to our investors concerning our ability to generate cash from our ongoing operating activities; by excluding these items, we believe we provide useful additional information to our investors to help them further understand our ability to generate cash period-over-period as well as added information on comparability to our competitors. Such as with Free Cash Flow information, as so adjusted, it is specifically not intended to provide amounts available for discretionary spending. We have added certain adjustments to account for items which we do not believe reflect our core business or operating performance, and we computed all periods with such adjusted costs.
Revenue at Constant Currency
To better understand trends in our business, we believe that it is helpful to adjust revenue to exclude the impact of changes in the translation of foreign currencies into U.S. Dollars. We refer to this adjusted revenue as “constant currency.” Currency impact is determined as the difference between actual growth rates and constant currency growth rates. This currency impact is calculated by translating the current period activity in local currency using the comparable prior-year period’s currency translation rate.
Non-GAAP Outlook
In providing the Full Year 2025 outlook for Adjusted EBITDA and Adjusted EBITDA Margin we exclude certain items which are otherwise included in determining the comparable U.S. GAAP financial measure. A description of the adjustments which historically have been applicable in determining Adjusted EBITDA and Adjusted EBITDA Margin is reflected in the table below. We are providing such outlook only on a non-GAAP basis because the company is unable without unreasonable efforts to predict with reasonable certainty the totality or ultimate outcome or occurrence of these adjustments for the forward-looking period, which can be dependent on future events that may not be reliably predicted. Based on past reported results, where one or more of these items have been applicable, such excluded items could be material, individually or in the aggregate, to reported results. We have provided an outlook for Adjusted Revenue only on a non-GAAP basis using foreign currency translation rates as of fiscal year end due to the inability to, without unreasonable efforts, accurately predict foreign currency impact on revenues. Full Year 2025 Outlook for Adjusted Free Cash Flow is provided as a factor of expected Adjusted EBITDA, and such outlook is only available on a non-GAAP basis for the reasons described above. For the same reason, we are unable to provide a GAAP expected adjusted tax rate, which adjusts for our non-GAAP adjustments.
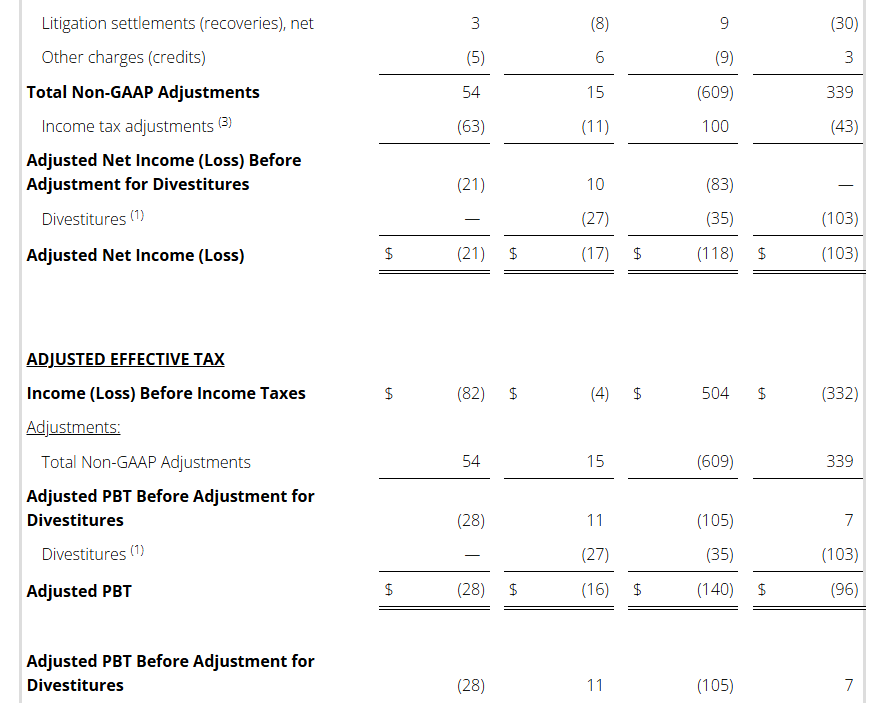
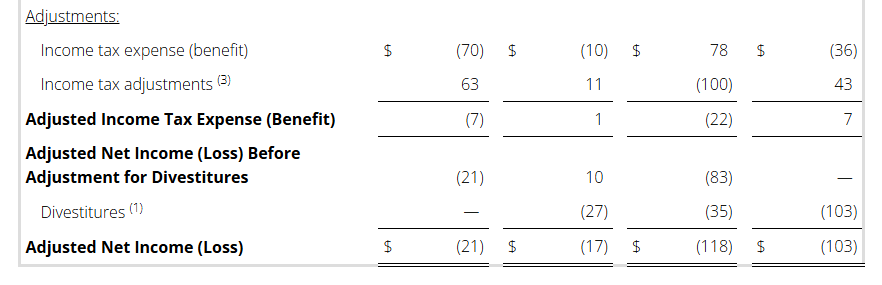
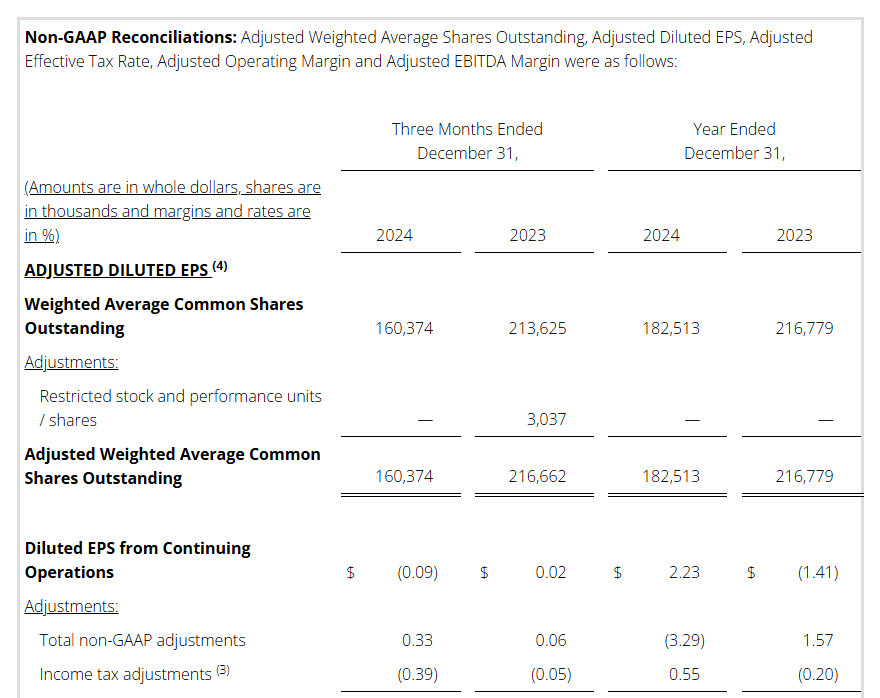
Non-GAAP Reconciliations: Adjusted Revenue, Revenue at Constant Currency, Adjusted Net Income (Loss), Adjusted Effective Tax, Adjusted Operating Income (Loss) and Adjusted EBITDA were as follows (see footnotes on last page of Non-GAAP reconciliations):
Media Contacts
Sean Collins
Conduent
+1-310-497-9205
Giles Goodburn
Conduent
+1-203-216-3546
















