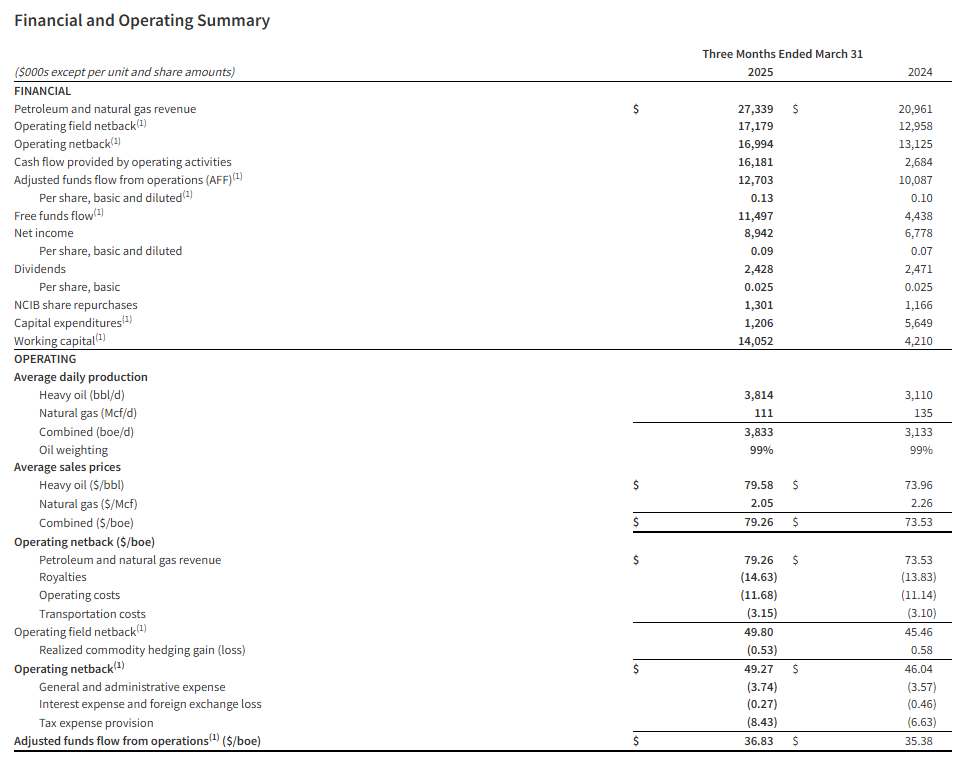
Research News and Market Data on LODE
Closes Initial $20 Million Tranche of Series A Investment and Separation of Fuels
VIRGINIA CITY, NEVADA, MAY 22, 2025 – Comstock Inc. (NYSE American: LODE) (“Comstock” and the “Company”) today announced that its executive chairman and chief executive officer, Corrado De Gasperis, issued the following letter to shareholders announcing major transformative milestones, including the separation of Comstock Fuels.
Dear Shareholders:
On behalf of our Board of Directors, Executive Officers, and the entire Comstock team, thank you for your continued support of our goals and bold strategies for achieving systemic decarbonization, establishing technological leadership in the massive global renewable fuels and renewable metals markets and positioning great value for all of our shareholders.
I am genuinely thrilled to announce the completion of the successful separation of our renewable fuels segment into a new independent entity, Bioleum Corporation (“Bioleum”), and its high-value capitalization, through the closing on the first $20 million in direct Series A equity investment. This investment is the first of $50 million planned this year.
This achievement fulfills the first phase of the plans we outlined earlier this year and positions us with two high-growth companies – one focused on renewable metals and mining here in Nevada, and the other on renewable fuels headquartered in Oklahoma – enabling two extremely different business and capital profiles and providing each the platform to thrive in their own markets. In doing so, we are unleashing unprecedented opportunities for growth and value creation.
Bioleum Separation Completed: A Bold New Chapter
We have officially separated and contributed the assets that formerly comprised Comstock’s fuels segment into Bioleum, a newly formed company dedicated to accelerating and maximizing the production and use of lignocellulosic biomass – or BioleumTM – derived fuels and attracting the necessary capitalization to do so. Bioleum’s formation marks the next chapter in this rapid evolution – one where our renewable fuels platform can accelerate under its own banner with singular focus and clarity with the ultimate objective of also becoming a publicly traded company.
Comstock now owns $65 million of the preferred stock in Bioleum, convertible into 32.5 million common shares, nearly the exact number of total outstanding shares of LODE today. This represents the substantial majority of Bioleum, positioning an exceptional value for our shareholders today and preserving our ability to accelerate the growth of that value and the delivery of that value directly to our shareholders, ultimately through a future public offering and beyond.
This transaction was structured to give Bioleum operational independence, with direct governance from Comstock and the strategic Series A investors, while maintaining and ensuring exceptional alignment with Comstock’s interests. Bioleum now has its own dedicated leadership and resources: Kevin Kreisler has been appointed Chief Executive Officer of Bioleum, and – in addition to my continuing role as Executive Chairman and Chief Executive Officer of Comstock, I will also serve as Bioleum’s Executive Chairman and Chief Financial Officer to both guide and support the new enterprise. I will be the only professional employed directly by both companies. With an independent board and governance, including representatives of the Series A investors and independent board members of Comstock, Bioleum can effectively and efficiently accelerate its mission and critical financing strategy. We believe that this structure, combined with Comstock’s ongoing governance and preferred equity interest, strikes the ideal balance to maximize Bioleum’s potential and unlock the full value of its clean energy portfolio for all of our shareholders.
Critically, the separation and its aligned structure, satisfies key conditions of large, sophisticated investors for new investment in Bioleum. Concurrently with the separation, Bioleum has closed an initial $20 million tranche of its Series A preferred stock financing. This infusion of growth capital validates Bioleum’s strategy and provides the resources to accelerate its next phase of development. These funds will support Bioleum’s continuing development as it completes engineering, financing, and construction of its first planned 400,000 barrel per year commercial demonstration facility in Oklahoma. In short, Bioleum is now equipped, capitalized, and structured to move forward at full speed. This capital also immediately relieves Comstock of a substantial majority of its liquidity and capital resource demands and constraints.
Bioleum’s Path to Full Commercialization
The initial objectives are clear. Bioleum will continue to focus on securing all approvals and agreements, including with top-tier Engineering, Procurement and Construction (“EPC”) and other strategic relationships while engineering is completed to construct its flagship 400,000 barrel per year refinery in Oklahoma. This first facility will deliver over $30 million in annual operating income once up and running, showcasing its commercial viability. Bioleum has already advanced site selection and engineering, and is working on project-level financing, permitting, regulatory approvals, and all other site agreements, fueled by the Series A capital.
Bioleum’s proprietary refining platform can convert underutilized woody biomass into a low-carbon, direct substitute for gasoline, diesel, and sustainable aviation fuel, effectively transforming waste biomass into high-value fuels with a carbon intensity as low as 15. In essence, we have uncovered a new kind of “oil well” – one that never runs dry because it is fed by renewable resources. As we often say, imagine an oil well that never stops producing – that is what Bioleum delivers. With market-leading yields of up to 140 gallons of fuel per ton of feedstock (on a gasoline gallon equivalent basis) and a platform designed to scale 10x beyond the first facility, Bioleum is poised to enlist major oil companies in accelerating and maximizing the production and use of Bioleum derived fuels.
Comstock Metals: Strength in Sustainable Innovation
While Bioleum separates, our Metals business will continue to grow stronger than ever. We are proud to report that Comstock Metals revenues are growing rapidly and achieving the highest levels of industry recognition. Recently, Comstock Metals became the first company in North America to earn the stringent R2v3 and RIOS certifications for zero-waste solar panel recycling. This groundbreaking certification validates that our recycling facility and processes meet the highest global standards for safety, environmental stewardship, and total waste elimination. In fact, under the R2v3/RIOS standard (including the specialized Appendix G), we demonstrated a 100% landfill-free recycling process – every component of an end-of-life solar panel (glass, aluminum, semiconductor fines, and other metals) is fully reclaimed and repurposed into new raw materials. Nothing goes to waste. This achievement is an extraordinary testament to our team’s innovation and diligence. It proves that our proprietary thermal recycling technology can deliver commodity-grade outputs from solar trash, with all parts of the panel fully recycled into valuable products.
Importantly, this certification also provides assurance to our customers, partners, and regulators that Comstock Metals’ operations meet the absolute highest bar for responsible recycling, without any reliance on government incentives. We have essentially built a new kind of mine above ground – one that harvests critical metals from retired solar panels instead of digging ore from the earth. As I’ve described before, it’s like a “world-class silver mine using solar panel waste as ore” that never depletes and just keeps on producing. With this validation in hand, Comstock Metals is aggressively scaling up its services to meet surging demand. We have been ramping up our recycling throughput (a fourfold increase year-over-year in Q1) and expanding our partnerships, including world-class customers and a master services agreement with RWE Clean Energy all while maintaining our zero-landfill promise. We are securing expanded and new permits and are preparing to expand to industry-scale operations at our Nevada facility to process dramatically larger volumes of photovoltaic waste. In short, Comstock Metals is now a proven leader in sustainable metal recycling, with a robust pipeline of business and technology that positions us for exponential growth. We could not be prouder of our teams.
Strategic Rationale: Capital Efficiency, Risk Management, and Value Creation
I want to clearly articulate our thinking behind separating the Bioleum and Comstock entities at this time. This transaction was driven by opportunity – and by necessity. Both our Fuels and Metals businesses have matured to a point where they can attract significant investment and growth on their own. However, they each have very different capital needs, risk profiles, and operational focuses. By separating them, we enable each company to pursue tailored funding and growth strategies with maximum efficiency. Bioleum can now raise capital (such as the Series A financing) directly into its projects and technology, without competing with or being bottlenecked by Comstock’s other needs. At the same time, Comstock Metals can seek its own strategic partnerships and financing faster and more efficiently (for new industry scale recycling facilities, for example) without having to dilute or divert resources to the Fuels business. This clear management, capital and growth focus in each company means more efficient use of funds and faster development cycles for both.
Separation also improves risk management. Renewable fuels and recyclable metals are adjacent in our mission, but they are fundamentally different businesses. Each business faces unique technical and market risks. By structurally isolating them, we ensure that challenges in one domain will not hinder the progress of the other. Investors and partners can now engage with a pure-play renewable metals producer or a pure-play renewable fuels developer, without ambiguity. This clarity reduces the perceived risk for stakeholders and allows each company to be evaluated on its own merits. Moreover, it grants each management team the autonomy to focus exclusively on its core mission – whether that is scaling up recycling operations or building biorefineries – thus sharpening execution and accountability.
Finally, and most importantly, this separation is about unlocking the full value and impact of the businesses we have built. We firmly believe that the combined value of Comstock and Bioleum as separate entities will exponentially exceed what might have been reflected as a single conglomerate. The market can now properly value our Metals business as the first mover in zero-waste solar recycling, and value Bioleum as a high-growth renewable fuels innovator, without one overshadowing the other. Early evidence of this value creation is clear: the separation and Bioleum’s financing were completed at an implied valuation that significantly uplifts the recognized value of our Fuels segment. Comstock retains a substantial majority equity position in Bioleum, so our shareholders keep substantial upside in Bioleum’s future success, while better protecting against downside risks and gaining all the benefits of a more focused parent company. We expect that as Bioleum reaches its milestones and as Comstock Metals rapidly expands, the sum-of-parts value to our shareholders will deliver dramatic and exponential returns.
Moving Forward
I want to emphasize the confidence I have in our bold path forward. We have spent the last four years investing in new technologies, overcoming challenges, and relentlessly driving toward commercialization of two revolutionary platforms. That effort has now culminated in a historic achievement: we launched Bioleum as an independent company and fortified Comstock Metals as a standalone powerhouse. We are now operating with a sharpened focus in each business. Our vision of enabling energy independence and a circular, decarbonized economy is not just alive – it’s accelerating.
Comstock emerges from this separation as a more streamlined enterprise laser-focused on renewable metals, with a healthy balance sheet and a stake in one of the most promising renewable fuel ventures in the world. Bioleum, for its part, is charging ahead to reinvent how we produce fuels, armed with world-class technology, a passionate team of industry veterans, and the funding to realize its plans. Together, though operating separately, Comstock and Bioleum are advancing complementary missions that address two of the world’s most pressing needs: clean energy and resource sustainability. We are leading – setting new standards, forging new partnerships, and capturing new markets.
I have never been more confident in our strategy or more excited looking to the future. We will continue executing with the same determination and ingenuity that brought us to this point. We look forward to creating extraordinary value together as we drive a true revolution in renewable fuels and sustainable materials.
Key Highlights
- Completed Separation of Comstock Fuels into Bioleum – Successfully separated our renewable fuels business into Bioleum Corporation, an independent entity, satisfying all conditions for the capital needs of the business. Comstock retains a substantial majority in Bioleum to participate in its tremendous growth potential.
- Comstock’s Stake – $65M Preferred Equity – Comstock received $65 million in preferred equity in Bioleum, convertible into 32.5 million common shares, representing a well-protected and substantial majority ownership of Bioleum. This ensures Comstock shareholders benefit from Bioleum’s future success.
- Bioleum Funded and Positioned for IPO – Bioleum closed an initial $20 million Series A financing concurrent with the separation. Proceeds will fund critical growth milestones. Bioleum plans an IPO upon completing the construction of its first 400,000 barrel per year refinery and meeting key commercialization milestones.
- Comstock Metals’ Extraordinary First Mover Advantage – Comstock Metals recycling business is thriving. Comstock Metals achieved the first R2v3/RIOS certification in North America for 100% zero-waste solar panel recycling, validating our world-class, zero-landfill process. Comstock Metals is scaling up with new permits, major customers, and expanding revenues, fulfilling the vision of a “solar waste silver mine” that never depletes.
- Strategic Benefits of Separation – The separation enhances capital efficiency (each company can raise and allocate capital optimally), improves risk management (distinct businesses are insulated from each other’s risks), and unlocks value (investors can properly value each pure-play business, driving greater aggregate shareholder value). We expect this separation to accelerate growth and maximize shareholder returns for both companies.
I appreciate everyone’s dedication and support in helping us get to this launching point. I am very much looking forward to discussing these achievements, our remarkable progress, and next steps with you during our 2025 Annual Meeting.
Kindest regards,
Corrado De Gasperis
Executive Chairman and Chief Executive Officer
Comstock Inc.
Additional information on the Bioleum transactions will be provided in Comstock’s planned Current Report on Form 8-K to be filed online on May 23, 2025.
About Bioleum Corporation
Bioleum Corporation (“Bioleum”) is setting a new standard in oil by developing and commercializing breakthrough refining technologies that convert lignocellulosic biomass into low-carbon transportation fuels at commercial scale, including cellulosic ethanol, gasoline, renewable diesel, synthetic aviation fuel (“SAF”), and other renewable fuels, with extremely low carbon intensity scores of 15 and market-leading yields of up to 140 gallons per dry metric ton of feedstock (on a gasoline gallon equivalent basis, or “GGE”). Bioleum plans to contribute to domestic energy dominance by directly building, owning, and operating a network of refineries in the U.S., starting with its planned first 400,000 barrel per year commercial demonstration facility in Oklahoma. Bioleum also licenses its advanced feedstock and refining solutions to third parties for additional production in the U.S. and global markets, including several recently announced and other pending projects. To learn more, please visit www.bioleum.com.
About Comstock Inc.
Comstock Inc. (NYSE: LODE) innovates and commercializes technologies that are deployable across entire industries to contribute to energy abundance by efficiently extracting and converting under-utilized natural resources, such as waste and other forms of woody biomass into renewable fuels, and end-of-life electronics into recovered electrification metals. To learn more, please visit www.comstock.inc.
Comstock Social Media Policy
Comstock Inc. has used, and intends to continue using, its investor relations link and main website at www.comstock.inc in addition to its X.com, LinkedIn and YouTube accounts, as means of disclosing material non-public information and for complying with its disclosure obligations under Regulation FD.
Contacts
For investor inquiries:
Judd B. Merrill, Chief Financial Officer
Tel (775) 413-6222
ir@comstockinc.com
For media inquiries:
Tracy Saville, Director of Marketing
Tel (775) 847-7573
media@comstockinc.com
Forward-Looking Statements
This press release and any related calls or discussions may include forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements, other than statements of historical facts, are forward-looking statements. The words “believe,” “expect,” “anticipate,” “estimate,” “project,” “plan,” “should,” “intend,” “may,” “will,” “would,” “potential” and similar expressions identify forward-looking statements but are not the exclusive means of doing so. Forward-looking statements include statements about matters such as: future market conditions; future explorations or acquisitions; divestitures, spin-offs or similar distribution transactions, future changes in our research, development and exploration activities; future financial, natural, and social gains; future prices and sales of, and demand for, our products and services; land entitlements and uses; permits; production capacity and operations; operating and overhead costs; future capital expenditures and their impact on us; operational and management changes (including changes in the Board of Directors); changes in business strategies, planning and tactics; future employment and contributions of personnel, including consultants; future land and asset sales; investments, acquisitions, divestitures, spin-offs or similar distribution transactions, joint ventures, strategic alliances, business combinations, operational, tax, financial and restructuring initiatives, including the nature, timing and accounting for restructuring charges, derivative assets and liabilities and the impact thereof; contingencies; litigation, administrative or arbitration proceedings; environmental compliance and changes in the regulatory environment; offerings, limitations on sales or offering of equity or debt securities, including asset sales and associated costs; business opportunities, growth rates, future working capital, needs, revenues, variable costs, throughput rates, operating expenses, debt levels, cash flows, margins, taxes and earnings. These statements are based on assumptions and assessments made by our management in light of their experience and their perception of historical and current trends, current conditions, possible future developments and other factors they believe to be appropriate. Forward-looking statements are not guarantees, representations or warranties and are subject to risks and uncertainties, many of which are unforeseeable and beyond our control and could cause actual results, developments, and business decisions to differ materially from those contemplated by such forward-looking statements. Some of those risks and uncertainties include the risk factors set forth in our filings with the SEC and the following: adverse effects of climate changes or natural disasters; adverse effects of global or regional pandemic disease spread or other crises; global economic and capital market uncertainties; the speculative nature of gold or mineral exploration, and lithium, nickel and cobalt recycling, including risks of diminishing quantities or grades of qualified resources; operational or technical difficulties in connection with exploration, metal recycling, processing or mining activities; costs, hazards and uncertainties associated with precious and other metal based activities, including environmentally friendly and economically enhancing clean mining and processing technologies, precious metal exploration, resource development, economic feasibility assessment and cash generating mineral production; costs, hazards and uncertainties associated with metal recycling, processing or mining activities; contests over our title to properties; potential dilution to our stockholders from our stock issuances, recapitalization and balance sheet restructuring activities; potential inability to comply with applicable government regulations or law; adoption of or changes in legislation or regulations adversely affecting our businesses; permitting constraints or delays; challenges to, or potential inability to, achieve the benefits of business opportunities that may be presented to, or pursued by, us, including those involving battery technology and efficacy, quantum computing and generative artificial intelligence supported advanced materials development, development of cellulosic technology in bio-fuels and related material production; commercialization of cellulosic technology in bio-fuels and generative artificial intelligence development services; ability to successfully identify, finance, complete and integrate acquisitions, spin-offs or similar distribution transactions, joint ventures, strategic alliances, business combinations, asset sales, and investments that we may be party to in the future; changes in the United States or other monetary or fiscal policies or regulations; interruptions in our production capabilities due to capital constraints; equipment failures; fluctuation of prices for gold or certain other commodities (such as silver, zinc, lithium, nickel, cobalt, cyanide, water, diesel, gasoline and alternative fuels and electricity); changes in generally accepted accounting principles; adverse effects of war, mass shooting, terrorism and geopolitical events; potential inability to implement our business strategies; potential inability to grow revenues; potential inability to attract and retain key personnel; interruptions in delivery of critical supplies, equipment and raw materials due to credit or other limitations imposed by vendors; assertion of claims, lawsuits and proceedings against us; potential inability to satisfy debt and lease obligations; potential inability to maintain an effective system of internal controls over financial reporting; potential inability or failure to timely file periodic reports with the Securities and Exchange Commission; potential inability to list our securities on any securities exchange or market or maintain the listing of our securities; and work stoppages or other labor difficulties. Occurrence of such events or circumstances could have a material adverse effect on our business, financial condition, results of operations or cash flows, or the market price of our securities. All subsequent written and oral forward-looking statements by or attributable to us or persons acting on our behalf are expressly qualified in their entirety by these factors. Except as may be required by securities or other law, we undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise. Neither this press release nor any related calls or discussions constitutes an offer to sell, the solicitation of an offer to buy or a recommendation with respect to any securities of the Company, the fund, or any other issuer.