
Research News and Market Data on GHM
- Revenue increased 7.3% to $47.0 million driven by continued strength in key end-markets
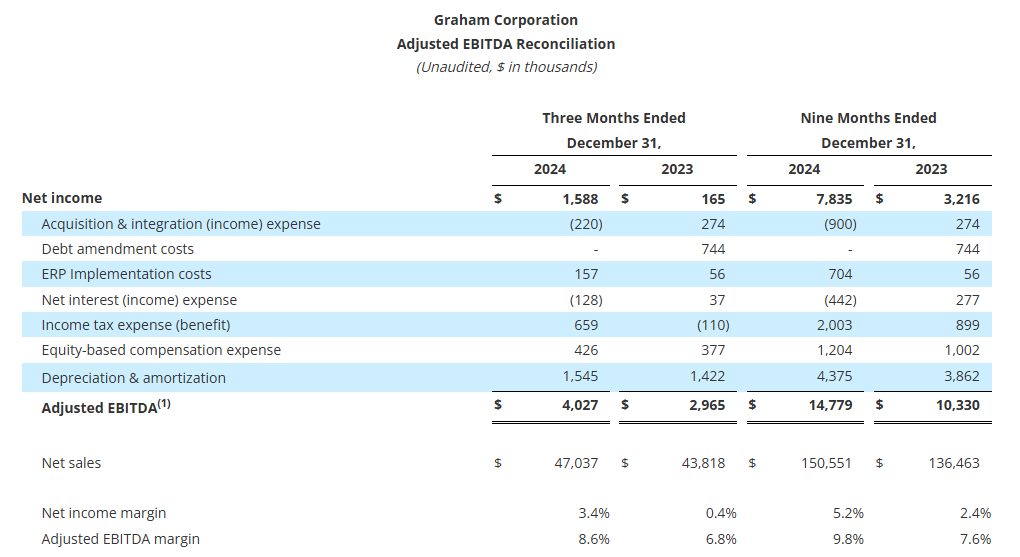
- Gross profit margin improved 260 basis points to 24.8% of sales, net margin increased 300 basis points to 3.4% of sales, and adjusted EBITDA margin1 expanded 180 basis points to 8.6% of sales
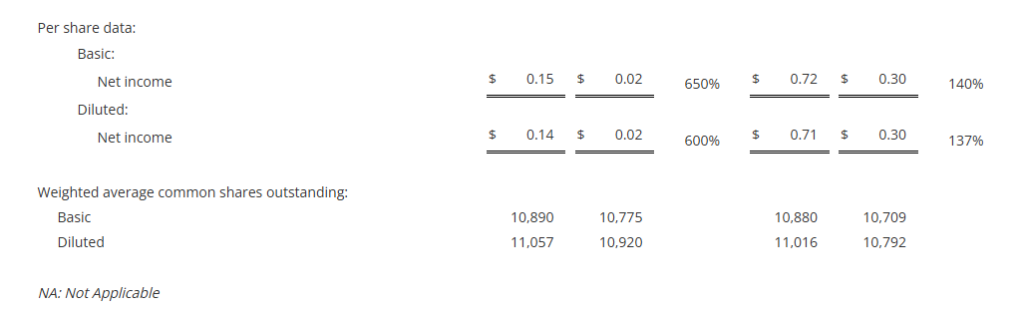
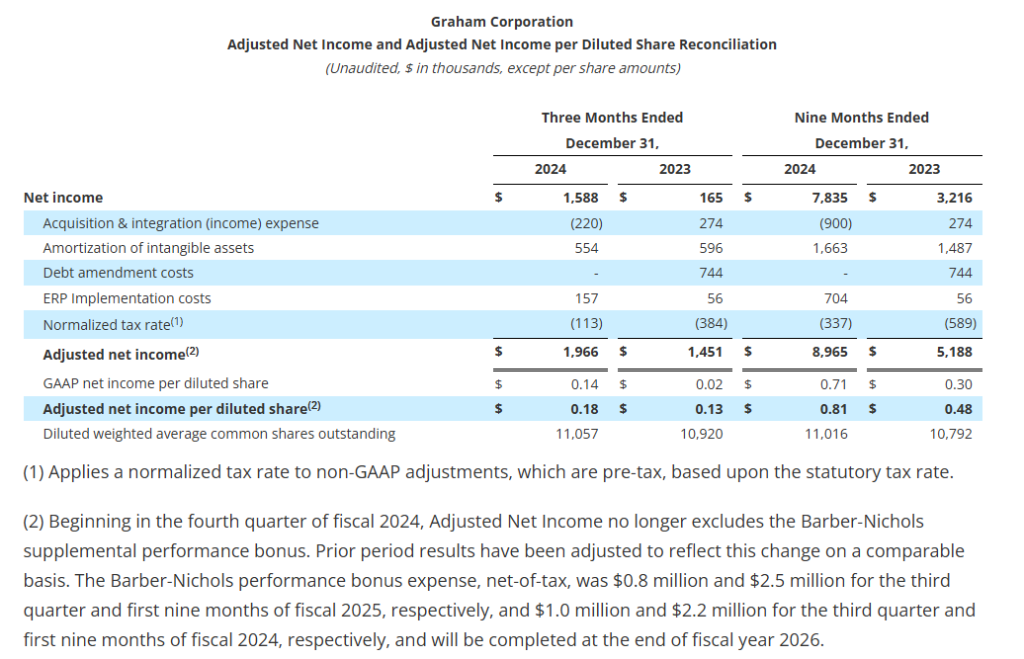
- Net income per diluted share increased 600% to $0.14 in the third quarter; adjusted net income per diluted share1 increased 38% to $0.18
- Orders of $24.8 million, driven by demand from defense, space, and aftermarket; YTD Book-to-Bill ratio of 1.0x and a backlog of $385 million2
- Strong balance sheet with no debt, $30.0 million in cash, and access to $43 million under its revolving credit facility at quarter end to support growth initiatives
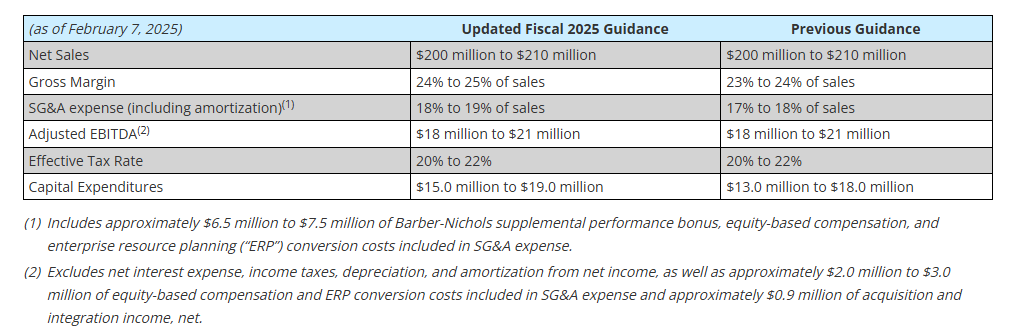
- Reiterated full year guidance for Sales and adjusted EBITDA1
BATAVIA, N.Y.–(BUSINESS WIRE)– Graham Corporation (NYSE: GHM) (“GHM” or the “Company”), a global leader in the design and manufacture of mission critical fluid, power, heat transfer and vacuum technologies for the defense, space, energy, and process industries, today reported financial results for its third quarter for the fiscal year ending March 31, 2025 (“fiscal 2025”).
“Our strong performance through the first three quarters of our fiscal year reflects continually improving execution across our business. Customer demand for our diversified product portfolio is robust, driving margin expansion through improved product mix and operational efficiency. The progress we have shown to date, coupled with advancing discussions on both new programs and expansions with existing customers, reinforces our confidence in achieving our long-term growth targets,” said Daniel J. Thoren, Chief Executive Officer.
____________________
1 Adjusted EBITDA margin, Adjusted Net Income per Diluted Share and Adjusted EBITDA are non-GAAP measures. See attached tables and other information for important disclosures regarding Graham’s use of these non-GAAP measures.
2 Orders, backlog and book-to-bill ratio are key performance metrics. See “Key Performance Indicators” below for important disclosures regarding Graham’s use of these metrics.
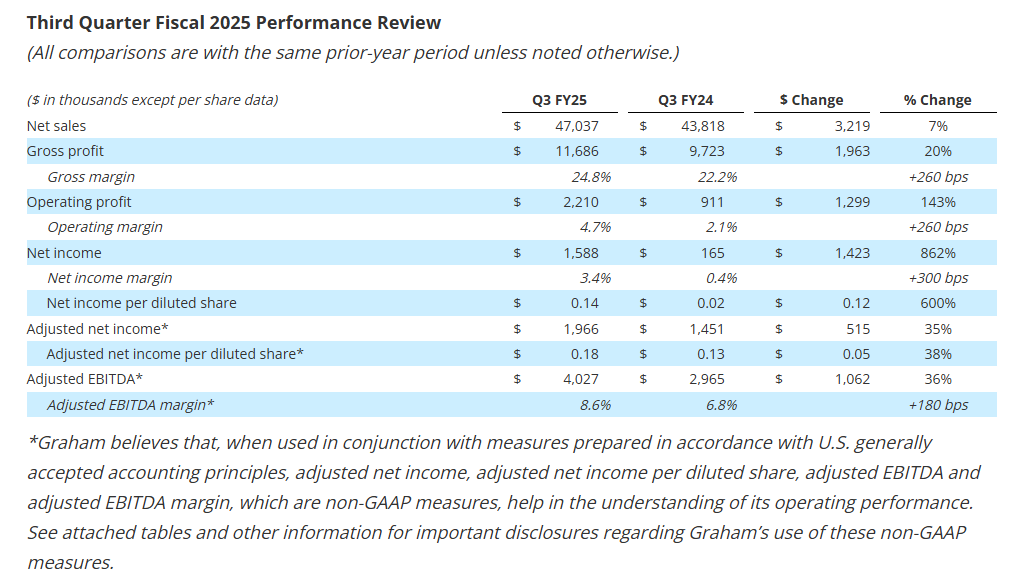
Quarterly net sales of $47.0 million increased 7.3%, or $3.2 million. Sales to the defense market grew by $2.7 million, or 11.1% from the prior year period, driven by the addition of new defense programs, the ramp-up of existing programs, better execution, and the timing of key project milestones. Additionally, higher chemical/petrochemical sales contributed $2.7 million to growth, driven by increased sales of capital equipment. Aftermarket sales to the refining, chemical/petrochemical, and defense markets of $9.7 million remained strong and were 2.4% higher than the prior year. See supplemental data for a further breakdown of sales by market and region.
Gross profit for the quarter increased $2.0 million to $11.7 million compared to the prior-year period of $9.7 million. As a percentage of sales, gross profit margin increased 260 basis points to 24.8%, compared to the fiscal third quarter of 2024. This increase was driven by leverage on higher volume, better execution, and improved pricing, partially offset by higher incentive compensation compared to the prior year period.
Additionally, the third quarter of fiscal 2025 gross profit benefited $0.3 million from a $2.1 million grant received from the BlueForge Alliance earlier this fiscal year to reimburse Graham for the cost of the Company’s defense welder training programs in Batavia and related equipment. To date, the Company has received $1.5 million of funding under this grant.
Selling, general and administrative expense (“SG&A”), including amortization, totaled $9.7 million, or 20.6% of sales, up $0.9 million compared with the prior year. This increase reflects the Company’s continued investments in its people, processes, and technology to drive long-term sustainable growth.
Included in other operating income for the third quarter of fiscal 2025 was a $0.2 million reversal of a previously accrued contingent earnout liability for P3. The reversal was due to delayed orders/projects that extended beyond the earnout period.
Cash Management and Balance Sheet
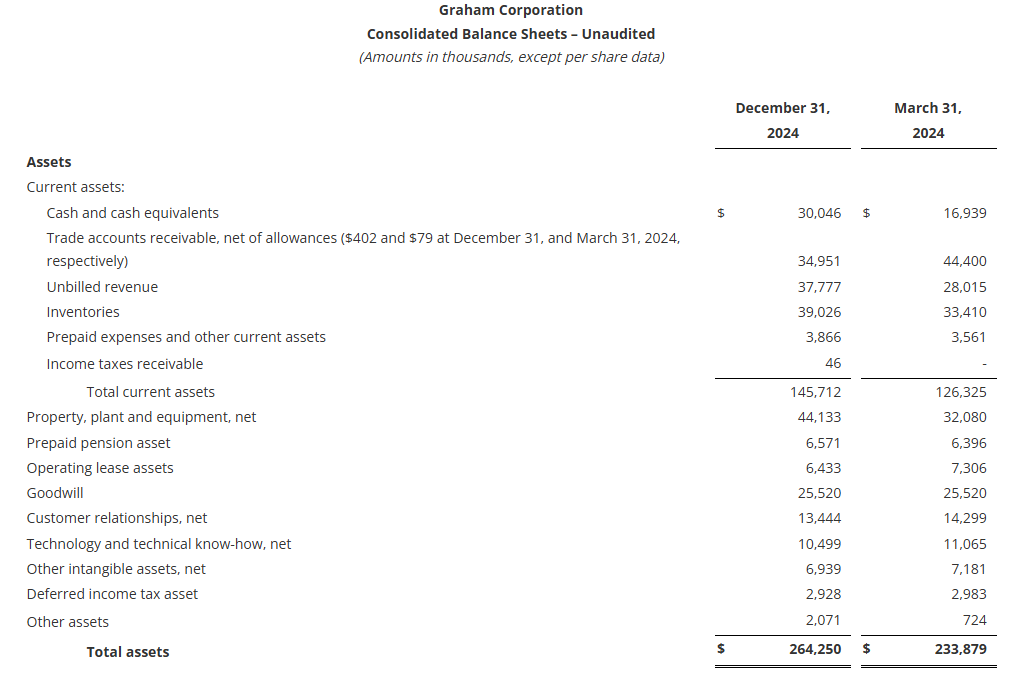
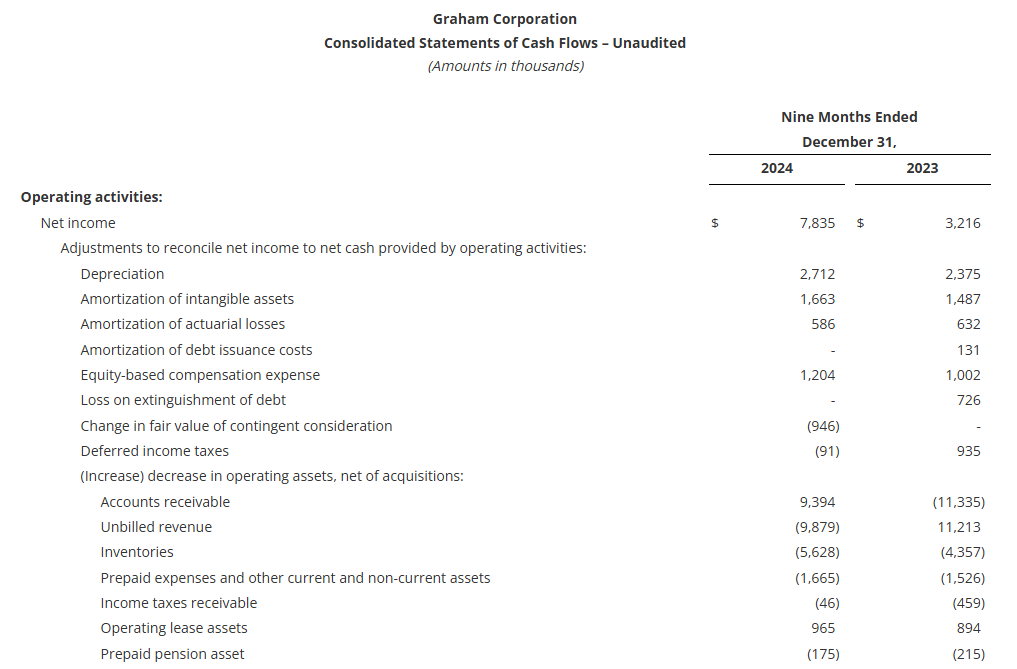
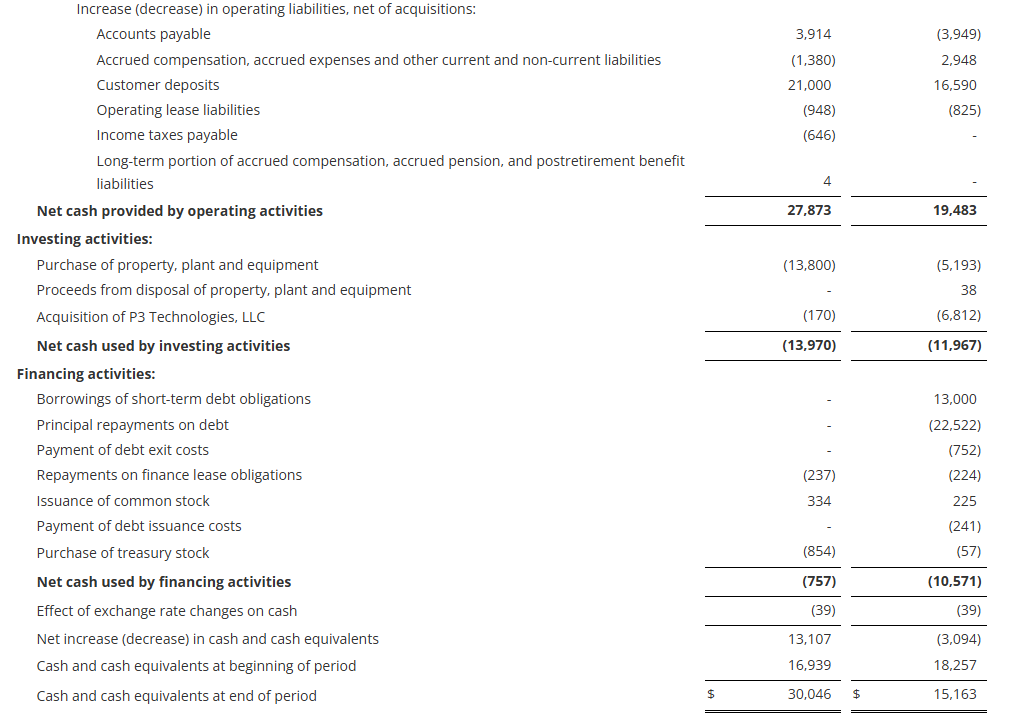
Cash provided by operating activities totaled $27.9 million for the nine-month period ending December 31, 2024, an increase of $8.4 million from the comparable period in fiscal 2024. As of December 31, 2024, cash and cash equivalents were $30.0 million, up from $16.9 million at the end of fiscal 2024.
Capital expenditures of $13.8 million for the first nine months of fiscal 2025 were focused on capacity expansion, increasing capabilities, and productivity improvements. The Company increased its expected fiscal 2025 capital expenditures to be in the range of $15.0 million to $19.0 million from its previous expectations of $13.0 million to $18.0 million due to a faster pace of execution on the capital projects in process. All major capital projects are on time and on budget.
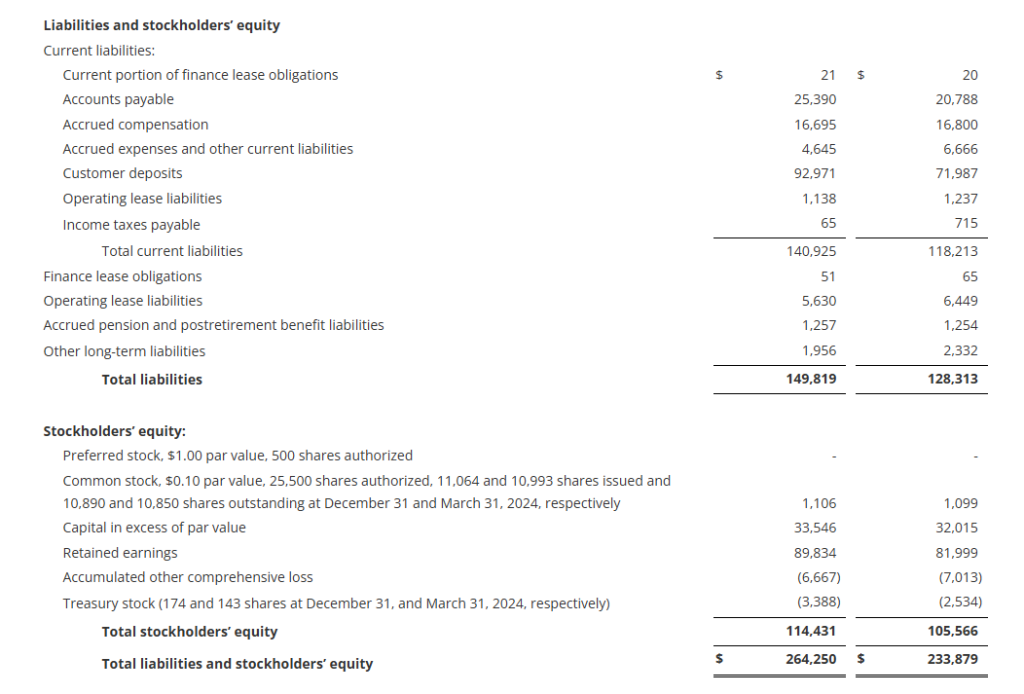
The Company had no debt outstanding at December 31, 2024 with $43 million available on its revolving credit facility after taking into account outstanding letters of credit.
Orders, Backlog, and Book-to-Bill Ratio
See supplemental data filed with the Securities and Exchange Commission on Form 8-K and provided on the Company’s website for a further breakdown of orders and backlog by market. See “Key Performance Indicators” below for important disclosures regarding Graham’s use of these metrics.
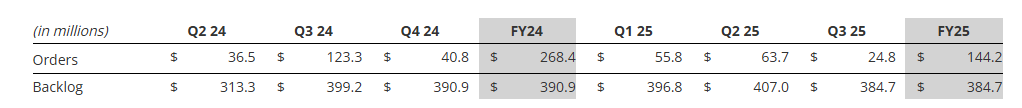
As expected, orders for the third quarter of fiscal 2025 declined to $24.8 million given the higher level of orders earlier in the fiscal year. Orders tend to be lumpy given the nature of our business (i.e. large capital projects) and in particular, orders to the defense industry, which span multiple years and are larger in size. Orders for the nine-month period ended December 31, 2024, were $144.2 million, resulting in a year-to-date book-to-bill ratio of 1.0x. After-market orders for the refining, petrochemical, and defense markets remained strong and totaled $13.0 million for the third quarter of fiscal 2025, an increase of 51% over the prior year.
Backlog at quarter end was $384.7 million, down 3.6% over the prior-year period and down 5.5% sequentially. Approximately 45% to 50% of orders currently in backlog are expected to be converted to sales in the next twelve months and another 35% to 40% are expected to convert to sales within one to two years. The majority of orders expected to convert beyond twelve months are for the defense industry, specifically the U.S. Navy.
Fiscal 2025 Outlook
The Company’s outlook for 2025 was updated as follows:
Webcast and Conference Call
GHM’s management will host a conference call and live webcast on February 7, 2025 at 11:00 a.m. Eastern Time (“ET”) to review its financial results as well as its strategy and outlook. The review will be accompanied by a slide presentation, which will be made available immediately prior to the conference call on GHM’s investor relations website.
A question-and-answer session will follow the formal presentation. GHM’s conference call can be accessed by calling (201) 689-8560. Alternatively, the webcast can be monitored from the events section of GHM’s investor relations website.
A telephonic replay will be available from 3:00 p.m. ET today through Friday, February 14, 2025. To listen to the archived call, dial (412) 317-6671 and enter conference ID number 13750971 or access the webcast replay via the Company’s website at ir.grahamcorp.com, where a transcript will also be posted once available.
About Graham Corporation
Graham is a global leader in the design and manufacture of mission critical fluid, power, heat transfer and vacuum technologies for the defense, space, energy, and process industries. Graham Corporation and its family of global brands are built upon world-renowned engineering expertise in vacuum and heat transfer, cryogenic pumps, and turbomachinery technologies, as well as its responsive and flexible service and the unsurpassed quality customers have come to expect from the Company’s products and systems. Graham Corporation routinely posts news and other important information on its website, grahamcorp.com, where additional information on Graham Corporation and its businesses can be found.
Safe Harbor Regarding Forward Looking Statements
This news release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended.
Forward-looking statements are subject to risks, uncertainties and assumptions and are identified by words such as “expects,” “future,” “outlook,” “anticipates,” “believes,” “could,” “guidance,” ”may”, “will,” “plan” and other similar words. All statements addressing operating performance, events, or developments that Graham Corporation expects or anticipates will occur in the future, including but not limited to, profitability of future projects and the business, its ability to deliver to plan, its ability to continue to strengthen relationships with customers in the defense industry, its ability to secure future projects and applications, expected expansion and growth opportunities, anticipated sales, revenues, adjusted EBITDA, adjusted EBITDA margins, capital expenditures and SG&A expenses, the timing of conversion of backlog to sales, orders, market presence, profit margins, tax rates, foreign sales operations, customer preferences, changes in market conditions in the industries in which it operates, changes in general economic conditions and customer behavior, forecasts regarding the timing and scope of the economic recovery in its markets, and its acquisition and growth strategy, are forward-looking statements. Because they are forward-looking, they should be evaluated in light of important risk factors and uncertainties. These risk factors and uncertainties are more fully described in Graham Corporation’s most recent Annual Report filed with the Securities and Exchange Commission (the “SEC”), included under the heading entitled “Risk Factors”, and in other reports filed with the SEC.
Should one or more of these risks or uncertainties materialize or should any of Graham Corporation’s underlying assumptions prove incorrect, actual results may vary materially from those currently anticipated. In addition, undue reliance should not be placed on Graham Corporation’s forward-looking statements. Except as required by law, Graham Corporation disclaims any obligation to update or publicly announce any revisions to any of the forward-looking statements contained in this news release.
Non-GAAP Financial Measures
Adjusted EBITDA is defined as consolidated net income (loss) before net interest expense, income taxes, depreciation, amortization, other acquisition related expenses, and other unusual/nonrecurring expenses. Adjusted EBITDA margin is defined as Adjusted EBITDA as a percentage of sales. Adjusted EBITDA and Adjusted EBITDA margin are not measures determined in accordance with generally accepted accounting principles in the United States, commonly known as GAAP. Nevertheless, Graham believes that providing non-GAAP information, such as Adjusted EBITDA and Adjusted EBITDA margin, is important for investors and other readers of Graham’s financial statements, as it is used as an analytical indicator by Graham’s management to better understand operating performance. Moreover, Graham’s credit facility also contains ratios based on Adjusted EBITDA. Because Adjusted EBITDA and Adjusted EBITDA margin are non-GAAP measures and are thus susceptible to varying calculations, Adjusted EBITDA, and Adjusted EBITDA margin, as presented, may not be directly comparable to other similarly titled measures used by other companies.
Adjusted net income and adjusted net income per diluted share are defined as net income and net income per diluted share as reported, adjusted for certain items and at a normalized tax rate. Adjusted net income and adjusted net income per diluted share are not measures determined in accordance with GAAP, and may not be comparable to the measures as used by other companies. Nevertheless, Graham believes that providing non-GAAP information, such as adjusted net income and adjusted net income per diluted share, is important for investors and other readers of the Company’s financial statements and assists in understanding the comparison of the current quarter’s and current fiscal year’s net income and net income per diluted share to the historical periods’ net income and net income per diluted share. Graham also believes that adjusted net income per share, which adds back intangible amortization expense related to acquisitions, provides a better representation of the cash earnings of the Company.
Forward-Looking Non-GAAP Measures
Forward-looking adjusted EBITDA and adjusted EBITDA margin are non-GAAP measures. The Company is unable to present a quantitative reconciliation of these forward-looking non-GAAP financial measures to their most directly comparable forward-looking GAAP financial measures because such information is not available, and management cannot reliably predict the necessary components of such GAAP measures without unreasonable effort largely because forecasting or predicting our future operating results is subject to many factors out of our control or not readily predictable. In addition, the Company believes that such reconciliations would imply a degree of precision that would be confusing or misleading to investors. The unavailable information could have a significant impact on the Company’s fiscal 2025 financial results. These non-GAAP financial measures are preliminary estimates and are subject to risks and uncertainties, including, among others, changes in connection with purchase accounting, quarter-end, and year-end adjustments. Any variation between the Company’s actual results and preliminary financial estimates set forth above may be material.
Key Performance Indicators
In addition to the foregoing non-GAAP measures, management uses the following key performance metrics to analyze and measure the Company’s financial performance and results of operations: orders, backlog, and book-to-bill ratio. Management uses orders and backlog as measures of current and future business and financial performance, and these may not be comparable with measures provided by other companies. Orders represent written communications received from customers requesting the Company to provide products and/or services. Backlog is defined as the total dollar value of net orders received for which revenue has not yet been recognized. Management believes tracking orders and backlog are useful as they often times are leading indicators of future performance. In accordance with industry practice, contracts may include provisions for cancellation, termination, or suspension at the discretion of the customer.
The book-to-bill ratio is an operational measure that management uses to track the growth prospects of the Company. The Company calculates the book-to-bill ratio for a given period as net orders divided by net sales.
Given that each of orders, backlog, and book-to-bill ratio are operational measures and that the Company’s methodology for calculating orders, backlog and book-to-bill ratio does not meet the definition of a non-GAAP measure, as that term is defined by the U.S. Securities and Exchange Commission, a quantitative reconciliation for each is not required or provided.


View source version on businesswire.com: https://www.businesswire.com/news/home/20250206161483/en/
Christopher J. Thome
Vice President – Finance and CFO
Phone: (585) 343-2216
Tom Cook
Investor Relations
(203) 682-8250
Tom.Cook@icrinc.com
Source: Graham Corporation
Released February 7, 2025


















