
Research News and Market Data on BTM
November 13, 2024 8:05 AM EST Download as PDF
ATLANTA, Nov. 13, 2024 (GLOBE NEWSWIRE) — Bitcoin Depot Inc. (“Bitcoin Depot” or the “Company”), a U.S.-based Bitcoin ATM operator and leading fintech company, today reported financial results for the third quarter ended September 30, 2024. Bitcoin Depot will host a conference call and webcast at 10:00 a.m. ET today. An earnings presentation and link to the webcast will be made available at ir.bitcoindepot.com.
“During the third quarter, we made significant strides in expanding our Bitcoin ATM network while working to optimize existing machines for greater profitability,” said Brandon Mintz, CEO and Founder of Bitcoin Depot. “We ended the quarter with 8,300 machines, surpassing our goals and reflecting our team’s execution and vision to enhance Bitcoin’s accessibility.
“In the past year, we’ve focused on relocating underperforming BTMs to more promising locations, a strategy that historically boosts average profitability per kiosk over time. While this improvement may not be fully visible in our consolidated quarterly numbers, the strategy is proving effective. With our extensive kiosk footprint and these initiatives in place, we are confident in our ability to drive shareholder value moving forward.
“As a result of our continued cash flow generation, we believe that beginning a cash dividend to common shareholders in 2025 will be one of the ways we create value for shareholders.”
Third Quarter 2024 Financial Results
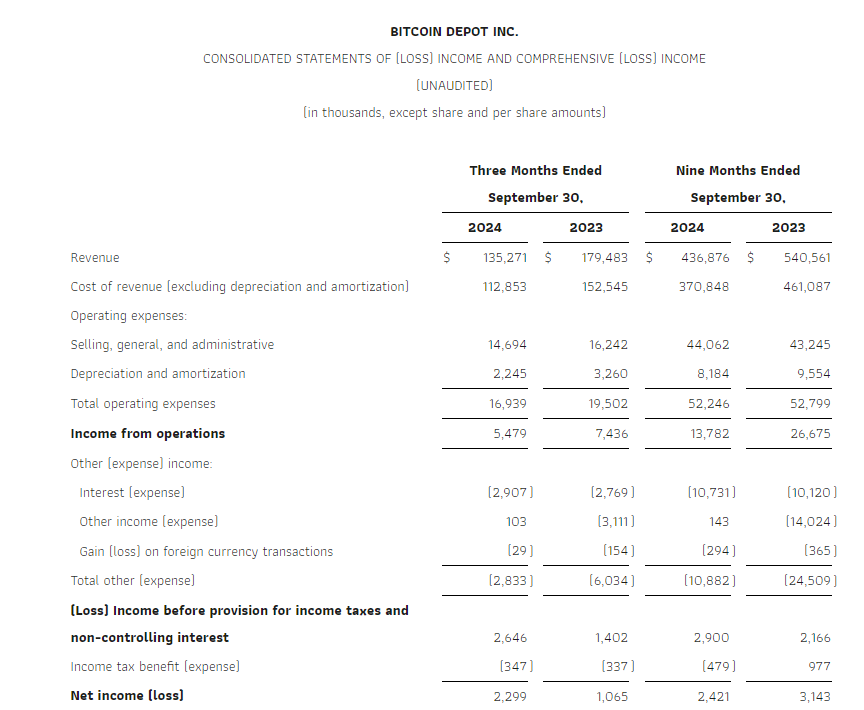
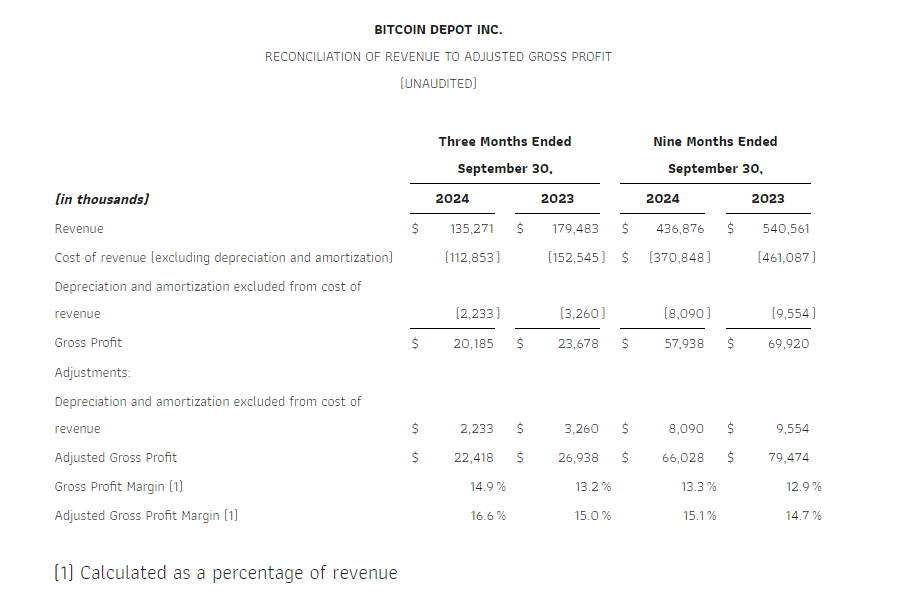
Revenue in the third quarter of 2024 was $135.3 million, down 25% from $179.5 million in the third quarter of 2023. This decline was largely driven by the impact of unfavorable legislation that was passed in California that went into effect in January 2024, along with the Company’s continued process of relocating underperforming kiosks in order to optimize fleet profitability.
Total operating expenses declined 13% to $16.9 million for the third quarter of 2024, compared to $19.5 million for the third quarter of 2023 due to costs of going public in 2023 that did not recur in 2024.
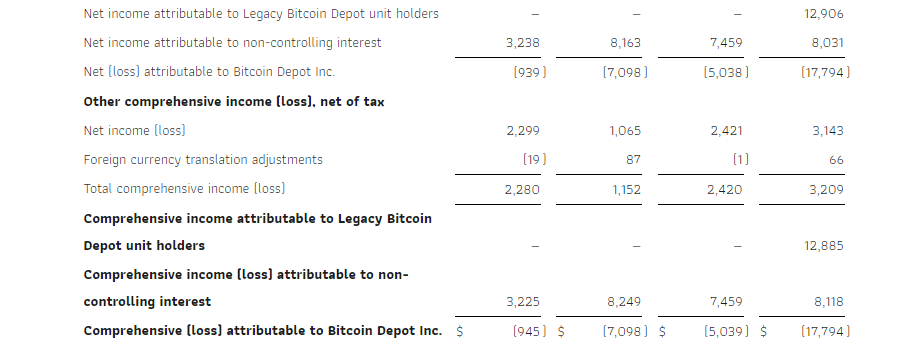
Net income for the third quarter of 2024 increased 116% to $2.3 million, compared to net income of $1.1 million for the third quarter of 2023. The increase was due to lower operating expenses in 2024, and expenses associated with the PIPE financing in the third quarter of 2023 that did not recur in 2024.
Adjusted gross profit, a non-GAAP measure, in the third quarter of 2024 was $22.4 million, down 17% from $26.9 million for the third quarter of 2023. Adjusted gross profit margin, a non-GAAP measure, in the third quarter of 2024 increased approximately 160 basis points to 16.6% compared to 15.0% in the third quarter of 2023. Please see “Explanation and Reconciliation of Non-GAAP Financial Measures” below.
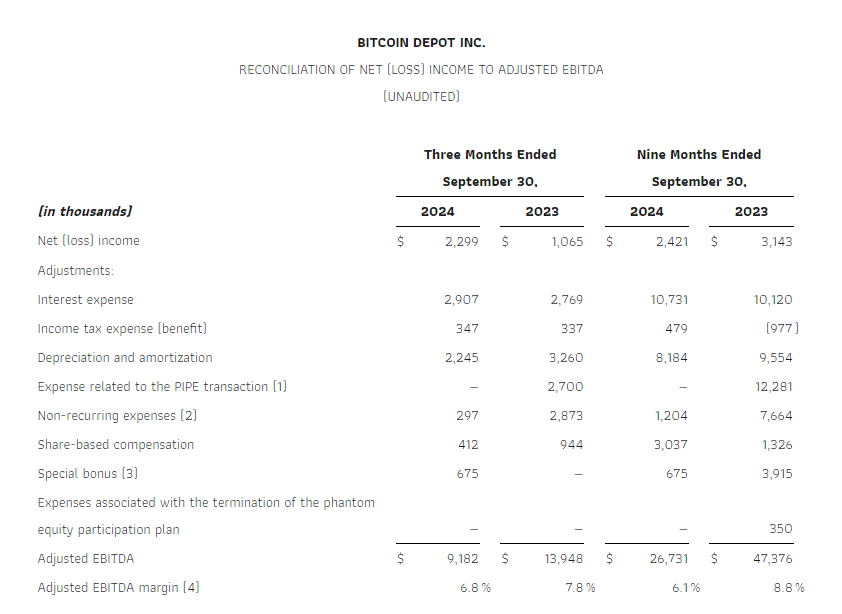
Adjusted EBITDA, a non-GAAP measure, in the third quarter of 2024 decreased 34% to $9.2 million, compared to Adjusted EBITDA of $13.9 million for the third quarter of 2023. The decline was due to the lower revenue. Please see “Explanation and Reconciliation of Non-GAAP Financial Measures” below.
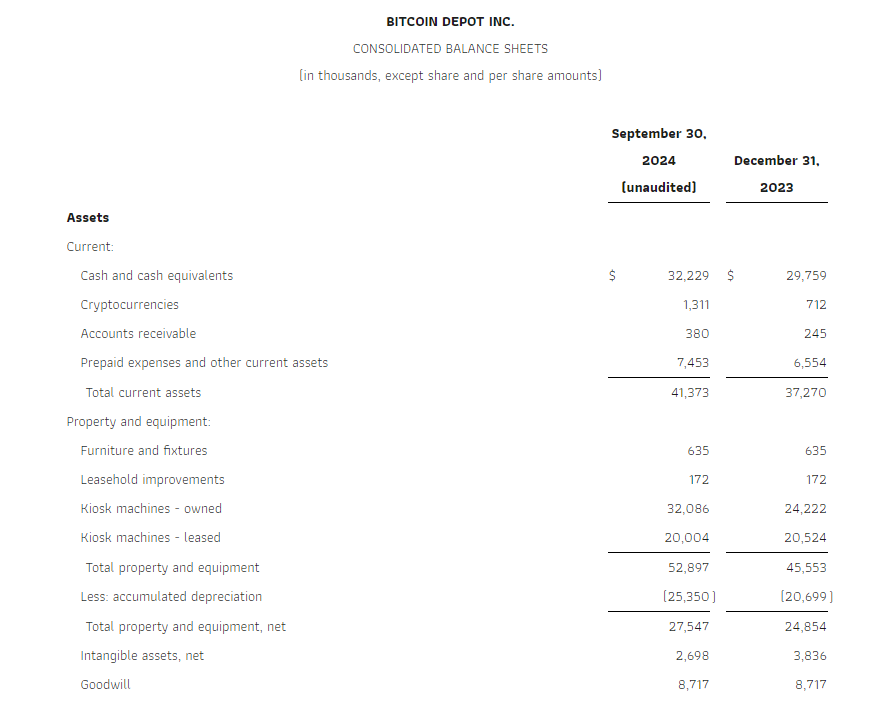
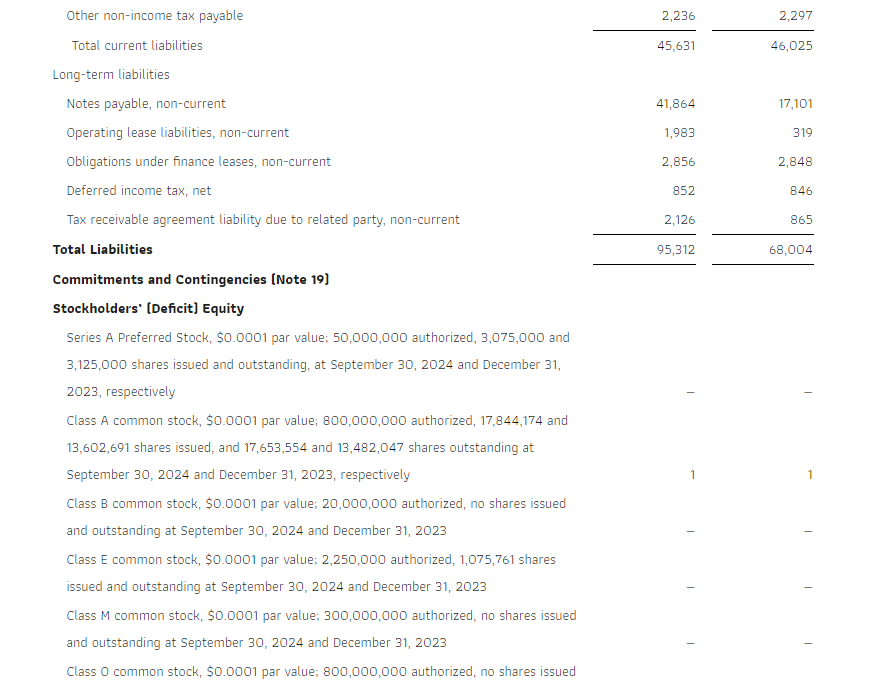
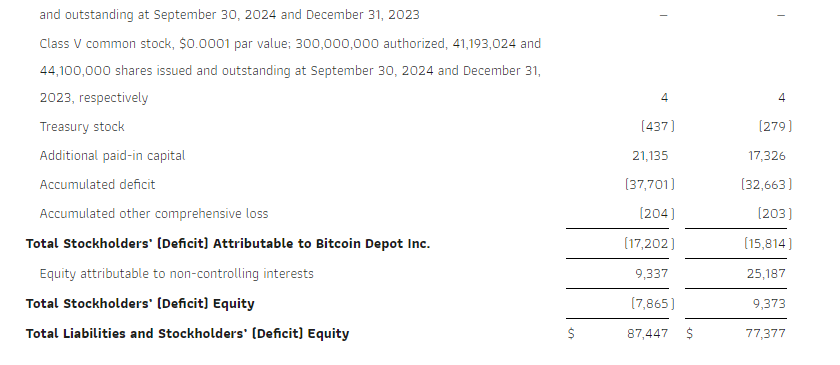
Cash and cash equivalents were $32.2 million as of the end of the third quarter of 2024. The Company generated $5.8 million in cash flows from operations in the third quarter and $17.3 million for the first nine months of 2024.
During the third quarter of 2024, the Company generated $5.8 million of cash flow from operations compared to $7.0 million in the third quarter of 2023.
Conference Call
Bitcoin Depot will hold a conference call at 10:00 a.m. Eastern time (7:00 a.m. Pacific time) today to discuss its financial results for the third quarter ended September 30, 2024.
Call Date: Wednesday, November 13, 2024
Time: 10:00 a.m. Eastern time (7:00 a.m. Pacific time)
U.S. dial-in: 646-968-2525
International dial-in: 888-596-4144
Conference ID: 7631242
The conference call will broadcast live and be available for replay here following the call.
Please call the conference telephone number approximately 10 minutes before the start time. An operator will register your name and organization. If you have any difficulty connecting with the conference call, please contact Bitcoin Depot’s investor relations team at 1-949-574-3860.
A replay of the call will be available beginning after 2:00 p.m. Eastern time on November 13, 2024, through November 20, 2024.
U.S. replay number: 609-800-9909
International replay number: 800-770-2030
Conference ID: 7631242
About Bitcoin Depot
Bitcoin Depot Inc. (Nasdaq: BTM) was founded in 2016 with the mission to connect those who prefer to use cash to the broader, digital financial system. Bitcoin Depot provides its users with simple, efficient and intuitive means of converting cash into Bitcoin, which users can deploy in the payments, spending and investing space. Users can convert cash to bitcoin at Bitcoin Depot kiosks in 48 states and at thousands of name-brand retail locations in 29 states through its BDCheckout product. The Company has the largest market share in North America with approximately 8,300 kiosk locations as of September 30, 2024. Learn more at www.bitcoindepot.com.
Cautionary Statement Regarding Forward-Looking Statements
This press release and any oral statements made in connection herewith include “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Exchange Act of 1934, as amended. Forward-looking statements are any statements other than statements of historical fact, and include, but are not limited to, statements regarding the expectations of plans, business strategies, objectives and growth and anticipated financial and operational performance, including our growth strategy and ability to increase deployment of our products and services, our ability to strengthen our financial profile, and worldwide growth in the adoption and use of cryptocurrencies. These forward-looking statements are based on management’s current beliefs, based on currently available information, as to the outcome and timing of future events. Forward-looking statements are often identified by words such as “anticipate,” “appears,” “approximately,” “believe,” “continue,” “could,” “designed,” “effect,” “estimate,” “evaluate,” “expect,” “forecast,” “goal,” “initiative,” “intend,” “may,” “objective,” “outlook,“ ”plan,“ ”potential,“ ”priorities,“ ”project,“ ”pursue,“ ”seek,“ ”should,“ ”target,“ ”when,“ ”will,“ ”would,” or the negative of any of those words or similar expressions that predict or indicate future events or trends or that are not statements of historical matters, although not all forward-looking statements contain such identifying words. In making these statements, we rely upon assumptions and analysis based on our experience and perception of historical trends, current conditions, and expected future developments, as well as other factors we consider appropriate under the circumstances. We believe these judgments are reasonable, but these statements are not guarantees of any future events or financial results. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on by any investor as, a guarantee, an assurance, a prediction or a definitive statement of fact or probability. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. Many actual events and circumstances are beyond our control.
These forward-looking statements are subject to a number of risks and uncertainties, including changes in domestic and foreign business, market, financial, political and legal conditions; failure to realize the anticipated benefits of the business combination; risks relating to the uncertainty of our projected financial information; future global, regional or local economic and market conditions; the development, effects and enforcement of laws and regulations; our ability to manage future growth; our ability to develop new products and services, bring them to market in a timely manner and make enhancements to our platform; the effects of competition on our future business; our ability to issue equity or equity-linked securities; the outcome of any potential litigation, government and regulatory proceedings, investigations and inquiries; and those factors described or referenced in filings with the Securities and Exchange Commission. If any of these risks materialize or our assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. There may be additional risks that we do not presently know or that we currently believe are immaterial that could also cause actual results to differ from those contained in the forward-looking statements. In addition, forward-looking statements reflect our expectations, plans or forecasts of future events and views as of the date of this press release. We anticipate that subsequent events and developments will cause our assessments to change.
We caution readers not to place undue reliance on forward-looking statements. Forward-looking statements speak only as of the date they are made, and we undertake no obligation to update publicly or otherwise revise any forward-looking statements, whether as a result of new information, future events, or other factors that affect the subject of these statements, except where we are expressly required to do so by law. All written and oral forward-looking statements attributable to us are expressly qualified in their entirety by this cautionary statement.

Explanation and Reconciliation of Non-GAAP Financial Measures
Bitcoin Depot reports its financial results in accordance with accounting principles generally accepted in the United States of America (“GAAP”). This press release includes both historical and projected Adjusted EBITDA, Adjusted Gross Profit, and certain ratios and other metrics derived therefrom such as Adjusted EBITDA margin and Adjusted Gross Profit margin, which are not prepared in accordance with GAAP.
Bitcoin Depot defines Adjusted EBITDA as net income before interest expense, income tax expense, depreciation and amortization, non-recurring expenses, share-based compensation, expenses related to the PIPE financing and miscellaneous cost adjustments. Such items are excluded from Adjusted EBITDA because these items are non-cash in nature, or because the amount and timing of these items is unpredictable, not driven by core results of operations and renders comparisons with prior periods and competitors less meaningful. In addition, Bitcoin Depot defines Adjusted Gross Profit (a non-GAAP financial measure) as revenue less cost of revenue (excluding depreciation and amortization) and depreciation and amortization adjusted to add back depreciation and amortization. Bitcoin Depot believes Adjusted EBITDA and Adjusted Gross Profit each provide useful information to investors and others in understanding and evaluating Bitcoin Depot’s results of operations, as well as provide a useful measure for period-to-period comparisons of Bitcoin Depot’s business performance. Adjusted EBITDA and Adjusted Gross Profit are each key measurements used internally by management to make operating decisions, including those related to operating expenses, evaluate performance and perform strategic and financial planning. However, you should be aware that Adjusted EBITDA and Adjusted Gross Profit are not measures of financial performance calculated in accordance with GAAP and may exclude items that are significant in understanding and assessing Bitcoin Depot’s financial results, and further, that Bitcoin Depot may incur future expenses similar to those excluded when calculating these measures. Bitcoin Depot primarily relies on GAAP results and uses both Adjusted EBITDA and Adjusted Gross Profit on a supplemental basis. Neither Adjusted EBITDA or Adjusted Gross Profit should be considered in isolation from, or as an alternative to, net income, cash flows from operations or other measures of profitability, liquidity or performance under GAAP and may not be indicative of Bitcoin Depot’s historical or future operating results. Bitcoin Depot’s computation of both Adjusted EBITDA and Adjusted Gross Profit may not be comparable to other similarly titled measures computed by other companies because not all companies calculate such measures in the same fashion. As such, undue reliance should not be placed on such measures.
Due to the high variability and difficulty in making accurate forecasts and projections of some of the information excluded from the projections of Adjusted EBITDA, together with some of the excluded information not being ascertainable or accessible, Bitcoin Depot is unable to quantify certain amounts that would be required to be included in the most directly comparable GAAP financial measures without unreasonable effort. Consequently, no disclosure of estimated comparable GAAP measures is included and no reconciliation of the forward-looking non-GAAP financial measures is included.
The following table presents a reconciliation of Net (loss) income to Adjusted EBITDA for the periods indicated:

Contacts:
Investors
Cody Slach,
Gateway Group, Inc.
949-574-3860
BTM@gateway-grp.com
Media
Zach Kadletz, Brenlyn Motlagh, Ryan Deloney
Gateway Group, Inc.
949-574-3860
BTM@gateway-grp.com

Source: Bitcoin Depot Inc.
Released November 13, 2024






















