Research News and Market Data on UNCY
January 29, 2026 9:15am EST Download as PDF
- FDA assigns Prescription Drug User Fee Act (PDUFA) target date of June 29, 2026
- Ended 2025 with unaudited cash position of $41.3M with expected runway into 2027
LOS ALTOS, Calif., Jan. 29, 2026 (GLOBE NEWSWIRE) — Unicycive Therapeutics, Inc. (“Unicycive” or the “Company”) (Nasdaq: UNCY), a clinical-stage biotechnology company developing therapies for patients with kidney disease, today announced that the U.S. Food and Drug Administration (FDA) has accepted the resubmission of its New Drug Application (NDA) for oxylanthanum carbonate (OLC), the Company’s investigational oral phosphate binder for the treatment of hyperphosphatemia in patients with chronic kidney disease (CKD) on dialysis. The Agency has deemed the OLC resubmission to be a Class II complete response which has a six-month review period from the date of resubmission and set a Prescription Drug User Fee Act (PDUFA) target action date of June 29, 2026.
“We are pleased that the agency has promptly accepted the resubmission of our NDA for OLC,” said Shalabh Gupta, M.D., Chief Executive Officer of Unicycive. “We are advancing our commercial preparation activities in anticipation of a potential launch of OLC later this year, to help provide an important treatment option to patients with chronic kidney disease (CKD) on dialysis who continue to struggle with hyperphosphatemia.”
The NDA is supported by data from three clinical studies (a Phase 1 study in healthy volunteers, a bioequivalence study in healthy volunteers and a tolerability study of OLC in CKD patients on dialysis), multiple preclinical studies as well as chemistry, manufacturing and controls (CMC) data. The FDA did not raise any concerns regarding OLC’s preclinical, clinical, or safety data included in the original NDA submission.
The Company ended 2025 with an unaudited position of $41.3 million in cash, cash equivalents, and short-term investments, which permits continued advancement of OLC commercial launch activities and a cash runway into 2027.
About Oxylanthanum Carbonate (OLC)
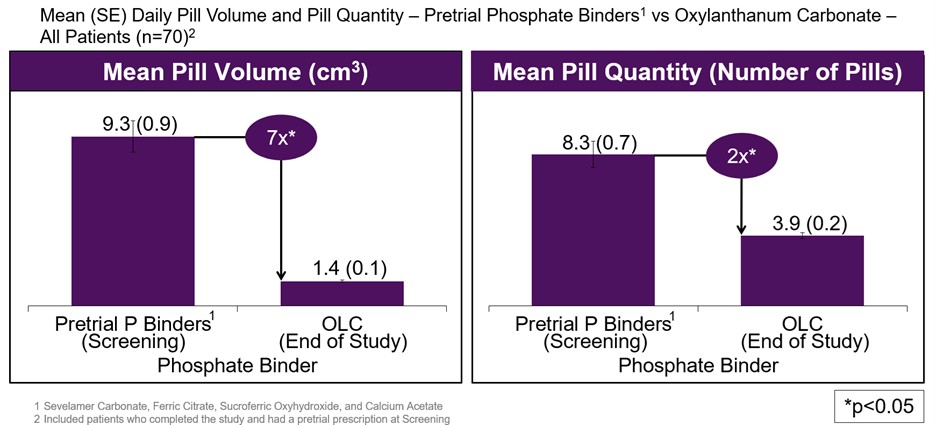
OLC is an investigational oral phosphate binder that leverages proprietary nanoparticle technology to deliver high phosphate binding potency, reducing the number and size of pills that patients must take to treat hyperphosphatemia in patients with chronic kidney disease (CKD) on dialysis. Its potential best-in-class profile may have meaningful patient adherence benefits over currently available treatment options as it requires a lower pill burden.
Unicycive is seeking FDA approval of OLC via the 505(b)(2) regulatory pathway. OLC is protected by a strong global patent portfolio including issued patents on composition of matter with exclusivity until 2031, and with the potential for patent term extension until 2035.
About Hyperphosphatemia
Hyperphosphatemia is a serious medical condition that occurs in nearly all patients with End Stage Renal Disease (ESRD). Annually there are over 450,000 individuals in the U.S. that require medication to control their phosphate levels.1 Uncontrolled hyperphosphatemia is strongly associated with increased death and hospitalization for CKD patients on dialysis. Treatment of hyperphosphatemia is aimed at lowering serum phosphate levels via two means: (1) restricting dietary phosphorus intake; and (2) using, on a daily basis, and with each meal, oral phosphate binding drugs that facilitate fecal elimination of dietary phosphate rather than its absorption from the gastrointestinal tract into the bloodstream.
1Flythe JE. Dialysis-Past, Present, and Future: A Kidney360 Perspectives Series. Kidney360. 2023 May 1;4(5):567-568. doi: 10.34067/KID.0000000000000145.
About Unicycive Therapeutics
Unicycive Therapeutics is a biotechnology company developing novel treatments for kidney diseases. Unicycive’s lead investigational treatment is oxylanthanum carbonate, a novel phosphate binding agent for the treatment of hyperphosphatemia in patients with chronic kidney disease who are on dialysis. Unicycive’s second investigational treatment UNI-494 is intended for the treatment of conditions related to acute kidney injury. It has been granted orphan drug designation (ODD) by the FDA for the prevention of Delayed Graft Function (DGF) in kidney transplant patients and has completed a Phase 1 dose-ranging safety study in healthy volunteers. For more information about Unicycive, visit Unicycive.com and follow us on LinkedIn and X.
Forward-looking statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified using words such as “anticipate,” “believe,” “forecast,” “estimated” and “intend” or other similar terms or expressions that concern Unicycive’s expectations, strategy, plans or intentions. These forward-looking statements are based on Unicycive’s current expectations and actual results could differ materially. There are several factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results; our clinical trials may be suspended or discontinued due to unexpected side effects or other safety risks that could preclude approval of our product candidates; risks related to business interruptions, which could seriously harm our financial condition and increase our costs and expenses; our need to raise substantial additional capital in the future to fund our continuing operations and the development and commercialization of our current product candidates and future product candidates; dependence on key personnel; substantial competition; uncertainties of patent protection and litigation; dependence upon third parties; risks related to delays in obtaining or failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; and our failure, or the failure of our third-party manufacturers, or their subcontractors, to comply with cGMPs or other applicable regulations, which could result in sanctions being imposed on us or the manufacturers, including fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates, operating restrictions and criminal prosecutions, any of which could adversely affect supplies of our product candidates and harm our business and results of operations. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: the uncertainties related to market conditions and other factors described more fully in the section entitled ‘Risk Factors’ in Unicycive’s Annual Report on Form 10-K for the year ended December 31, 2024, and other periodic reports filed with the Securities and Exchange Commission. Any forward-looking statements contained in this press release speak only as of the date hereof, and Unicycive specifically disclaims any obligation to update any forward-looking statement, whether as a result of new information, future events or otherwise.
Investor Contacts:
Kevin Gardner
LifeSci Advisors
kgardner@lifesciadvisors.com
Media Contact:
Layne Litsinger
Real Chemistry
llitsinger@realchemistry.com
SOURCE: Unicycive Therapeutics, Inc.

Source: Unicycive Therapeutics, Inc.
Released January 29, 2026