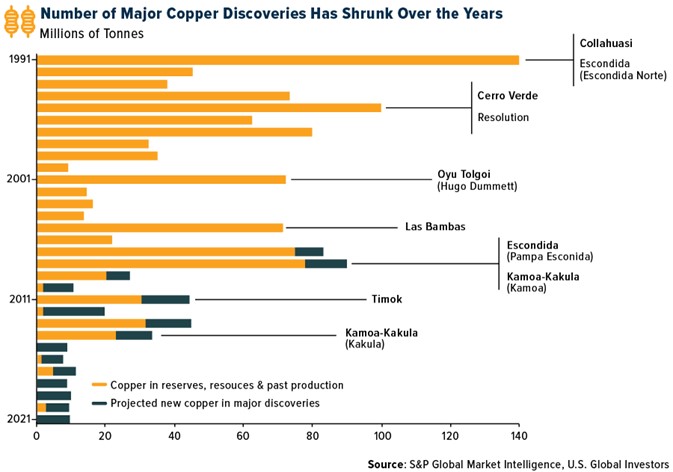
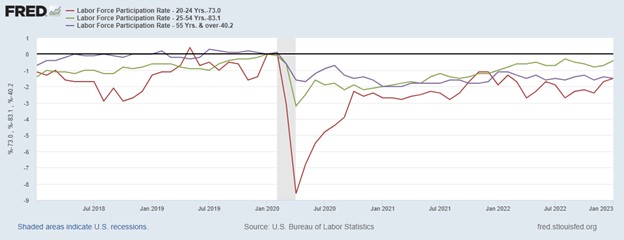
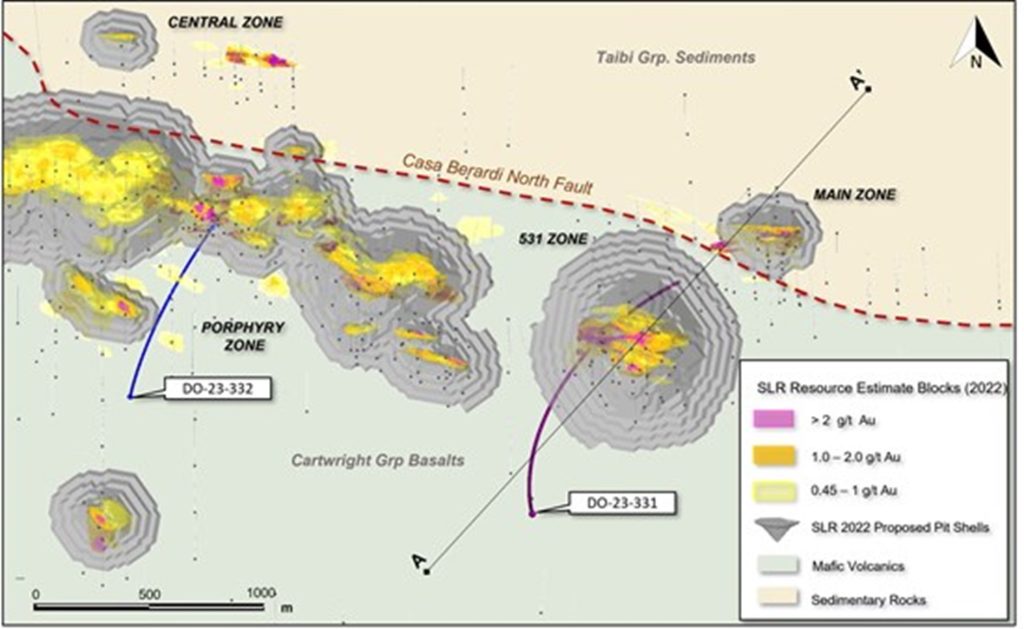
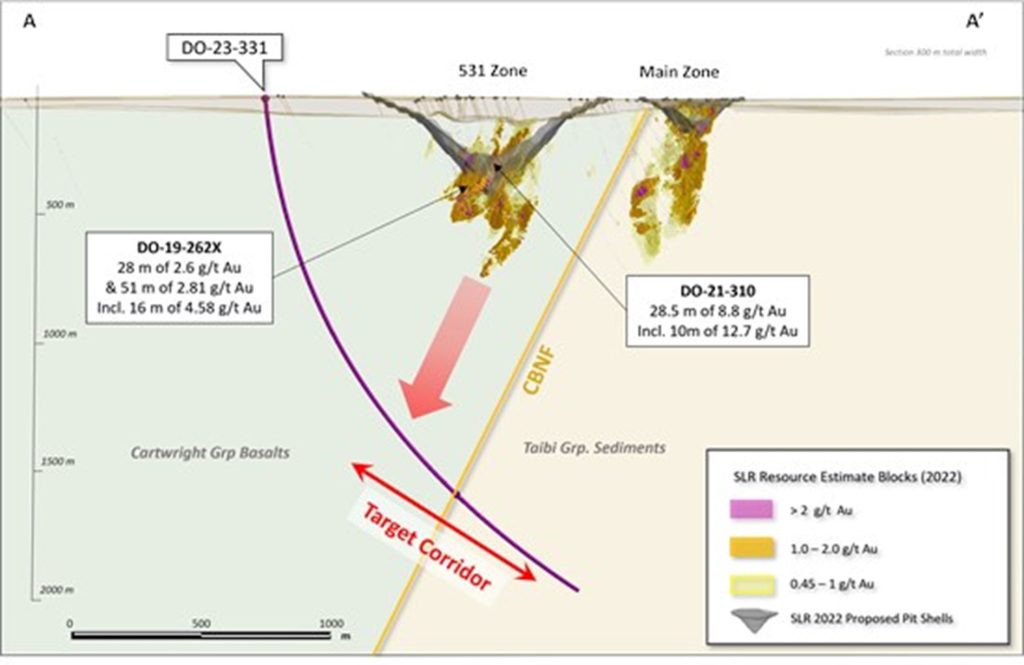
Research News and Market Data on TNXP
February 09, 2023 7:00am EST
Preclinical data demonstrate the efficacy of TNX‐801 vaccination against monkeypox virus challenge in an animal model
Phase 1 trial with TNX-801 for the prevention of monkeypox and smallpox is expected to start in the second half of 2023
CHATHAM, N.J, Feb. 09, 2023 (GLOBE NEWSWIRE) — Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP) (Tonix or the Company), a clinical-stage biopharmaceutical company, today announced the publication of a paper entitled, “Single Dose of Recombinant Chimeric Horsepox Virus (TNX‐801) Vaccination Protects Macaques from Lethal Monkeypox Challenge,” in the journal Viruses. The publication demonstrates that a single dose vaccination with TNX‐801 was effective at protecting non-human primates from infection with monkeypox virus. The article can be accessed online at https://www.mdpi.com/1999-4915/15/2/356.
“The global monkeypox outbreak that started in the spring of 2022 reinforced the importance of protecting the population using live virus vaccines,” said Seth Lederman, M.D., Chief Executive Officer of Tonix Pharmaceuticals. “Consequently, there is a need for an effective and safer, single dose, live replicating vaccine against monkeypox virus. Given the encouraging preclinical data to date, one potential approach is to use a live virus vaccine based on horsepox, such as TNX-801.”
David Evans, Ph.D., FCAHS, Professor and former Vice-Dean (Research) Faculty of Medicine and Dentistry at the University of Alberta and an investigator in the study and author of the publication, said, “It is often forgotten that vaccines don’t always produce sterilizing immunity and so it’s very exciting to be able to report that a horsepox-based vaccine works so well in such a challenging infection model.”
The publication describes data from animals in which eight out of eight vaccinated with TNX-801 were fully protected with sterilizing immunity from a challenge with intra-tracheal monkeypox (central African, or Congo Basin, clade). The vaccinations with TNX-801 were well tolerated as indicated by the lack of any overt clinical disease. These data show that the immunity generated by TNX‐801 was able to provide protection against a lethal challenge with monkeypox virus and is the first study to demonstrate the efficacy of TNX‐801 vaccination against monkeypox virus challenge in a non‐human primate model. Synthetic horsepox virus is the basis for the Company’s TNX-801 vaccine in development to protect against monkeypox and smallpox and for the Company’s Recombinant Pox Virus (RPV) platform to protect against other pathogens, including SARS-CoV-2.
About TNX-801 and TNX-1850
TNX-801 is a live virus vaccine based on horsepox2,3. Tonix is developing TNX-801 for percutaneous administration as a vaccine to protect against monkeypox and smallpox. Tonix is also developing TNX-1850 (horsepox-based live virus vaccine) for the prevention of COVID-19. TNX-1850 is designed to express the spike protein from the BA.2 variant of SARS-CoV-2. Tonix has previously reported positive data from a SARS-CoV-2 challenge study in non-human primates in which animals were vaccinated with TNX-1800, a horsepox-based vaccine expressing spike protein from the Wuhan strain4. Tonix’s TNX-801 is based on the sequence of the 1976 natural isolate Mongolian horsepox clone MNR-763.2 Molecular analysis of DNA sequences suggests that TNX-801 is closer than modern smallpox vaccines to the vaccine discovered and disseminated by Dr. Edward Jenner in 17985-7. For example, recent studies8,9 have shown approximately 99.7% colinear identity between TNX-801 and the circa 1860 U.S. smallpox vaccine VK05.10 The small plaque size in culture of TNX-801 appears identical to the U.S. Centers for Disease Control publication of the natural isolate11. Relative to vaccinia, horsepox has substantially decreased virulence in mice2. Dr. Edward Jenner invented vaccination in 1798 and the procedure was called “vaccination” because ‘cow’ is ‘vacca’ in Latin and the inoculum material was initially obtained from lesions on the udders of cows affected by a mild disease known as cowpox. However, Dr. Jenner suspected that cowpox originated from horses7. Subsequently, Dr. Jenner and others immunized against smallpox using material directly obtained from horses. The use of vaccines from horses was sometimes called ‘equination’ from the Latin ‘equus’ which means ‘horse’12. Equination and vaccination were practiced side-by-side in Europe12,13.
About the Recombinant Pox Virus (RPV) Platform
Horsepox virus and vaccines based on its use as a vector are live replicating viruses that elicit strong immune responses. Live replicating orthopoxviruses, like vaccinia or horsepox, can be engineered to express foreign genes and have been exploited as platforms for vaccine development because they possess; (1) large packaging capacity for exogenous DNA inserts, (2) precise virus-specific control of exogenous gene insert expression, (3) lack of persistence or genomic integration in the host, (4) strong immunogenicity as a vaccine, (5) ability to rapidly generate vector/insert constructs, (6) manufacturable at scale, and (7) ability to provide direct antigen presentation. Horsepox-based vaccines are designed to be single dose, vial-sparing vaccines, that can be manufactured using conventional cell culture systems, with the potential for mass scale production and packaging in multi-dose vials. Tonix’s TNX-801 and RPV vaccine candidates are administered percutaneously using a two-pronged, or “bifurcated” needle. The major cutaneous reaction or “take” to vaccinia vaccine was described by Dr. Edward Jenner in 1796 and has been used since then as a biomarker for protective immunity to smallpox, including in the World Health Organization’s (WHO) accelerated smallpox eradication program that successfully eradicated smallpox in the 1960’s. The “take” is a measure of functional T cell immunity validated by the eradication of smallpox, a respiratory-transmitted disease caused by variola.
About Monkeypox and Smallpox
Monkeypox14 and smallpox15 are diseases in humans caused by the monkeypox and smallpox (or variola) viruses, respectively. Monkeypox and variola are closely related orthopox viruses. Vaccination against smallpox with live virus vaccines based on horsepox or vaccinia protects against monkeypox. After routine smallpox vaccination was stopped in about 1970, monkeypox has become a growing problem in Africa. Since May of 2022, approximately 30,000 cases have been identified in the United States.16,17 There are two distinct clades of the monkeypox virus: the central African (Congo Basin) clade, and the west African clade which is associated with the recent outbreak. Historically, the Congo Basin clade has caused more severe disease than the west African clade. In recent times, the case fatality ratio for the virus is about 3–6%.18 In November, 2022, the WHO began using a new preferred term “mpox” as a synonym for monkeypox.19 Smallpox is considered eradicated, but there are concerns about malicious reintroduction.
Tonix Pharmaceuticals Holding Corp.1
Tonix is a clinical-stage biopharmaceutical company focused on discovering, licensing, acquiring and developing therapeutics to treat and prevent human disease and alleviate suffering. Tonix’s portfolio is composed of central nervous system (CNS), rare disease, immunology and infectious disease product candidates. Tonix’s CNS portfolio includes both small molecules and biologics to treat pain, neurologic, psychiatric and addiction conditions. Tonix’s lead CNS candidate, TNX-102 SL (cyclobenzaprine HCl sublingual tablet), is in mid-Phase 3 development for the management of fibromyalgia with a new Phase 3 study launched in the second quarter of 2022 and interim data expected in the second quarter of 2023. TNX-102 SL is also being developed to treat Long COVID, a chronic post-acute COVID-19 condition. Tonix initiated a Phase 2 study in Long COVID in the third quarter of 2022. TNX-1300 (cocaine esterase) is a biologic designed to treat cocaine intoxication and has been granted Breakthrough Therapy designation by the FDA. A Phase 2 study of TNX-1300 is expected to be initiated in the second quarter of 2023. TNX-1900 (intranasal potentiated oxytocin), a small molecule in development for chronic migraine, is being studied in a potential pivotal Phase 2 study that initiated enrollment in the first quarter of 2023. TNX-601 ER (tianeptine hemioxalate extended-release tablets) is a once-daily formulation of tianeptine being developed as a potential treatment for major depressive disorder (MDD) with a Phase 2 study expected to be initiated in the first quarter of 2023. Tonix’s rare disease portfolio includes TNX-2900 (intranasal potentiated oxytocin) for the treatment of Prader-Willi syndrome. TNX-2900 has been granted Orphan Drug designation by the FDA. Tonix’s immunology portfolio includes biologics to address organ transplant rejection, autoimmunity and cancer, including TNX-1500, which is a humanized monoclonal antibody targeting CD40-ligand (CD40L or CD154) being developed for the prevention of allograft and xenograft rejection and for the treatment of autoimmune diseases. A Phase 1 study of TNX-1500 is expected to be initiated in the second quarter of 2023. Tonix’s infectious disease pipeline includes a vaccine in development to prevent smallpox and monkeypox, TNX-801, a next-generation vaccine to prevent COVID-19, TNX-1850, a platform to make fully human monoclonal antibodies to treat COVID-19, TNX-3600, and humanized anti-SARS-CoV-2 monoclonal antibodies, TNX-3800, recently licensed from Curia. TNX-801, Tonix’s vaccine in development to prevent smallpox and monkeypox, also serves as the live virus vaccine platform or recombinant pox vaccine (RPV) platform for other infectious diseases. A Phase 1 study of TNX-801 is expected to be initiated in the second half of 2023.
1All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication.
2Noyce RS, et al. (2018) PLoS One. 13(1):e0188453
3Tulman ER, et al. (2006) J Virol. 80(18):9244-58.PMID:16940536
4Tonix Press Release March 16, 202a https://ir.tonixpharma.com/news-events/press-releases/detail/1255/tonix-pharmaceuticals-reports-positive-covid-19-vaccine
5Schrick L et al. (2017) N Engl J Med. 377:1491.
6Qin et al. (2015) J. Virol. 89:1809.
7Jenner E. “An Inquiry Into the Causes and Effects of the Variolae Vaccinae: A Disease Discovered in Some of the Western Counties of England, Particularly Gloucestershire, and Known by the Name of the Cow Pox.” London: Sampson Low, 1798.
8Brinkmann A et al, Genome Biology (2020) 21:286 https://doi.org/10.1186/s13059-020-02202-0
9Duggan A et al. Genome Biology (2020) 21:175 https://doi.org/10.1186/s13059-020-02079-z
10Tonix press release. Dec 4, 2020 https://ir.tonixpharma.com/news-events/press-releases/detail/1236/vaccine-genome-researchers-report-99-7-colinear-identity
11Trindale GS et al. (2016) Viruses (12). Pii: E328. PMID:27973399
12Esparza E, et al (2017) Vaccine. 35(52):7222-7230.
13Esparza J et al. (2020) Vaccine.; 38(30):4773-4779.
14www.cdc.gov/poxvirus/monkeypox/about.html
15www.cdc.gov/smallpox/research/
16Mandavilli, A. The New York Times. May 26, 2020. “Who is protected against monkeypox”
17 www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html – Accessed Feb 8, 2023
18 https://www.who.int/news-room/fact-sheets/detail/monkeypox#:~:text=There%20are%20two%20distinct%20genetic,thought%20to%20be%20more%20transmissible – Accessed Feb 8, 2023
19 https://www.who.int/news/item/28-11-2022-who-recommends-new-name-for-monkeypox-disease – Accessed Feb 8, 2023
This press release and further information about Tonix can be found at www.tonixpharma.com.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking words such as “anticipate,” “believe,” “forecast,” “estimate,” “expect,” and “intend,” among others. These forward-looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, risks related to the failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID-19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. Tonix does not undertake an obligation to update or revise any forward-looking statement. Investors should read the risk factors set forth in the Annual Report on Form 10-K for the year ended December 31, 2021, as filed with the Securities and Exchange Commission (the “SEC”) on March 14, 2022, and periodic reports filed with the SEC on or after the date thereof. All of Tonix’s forward-looking statements are expressly qualified by all such risk factors and other cautionary statements. The information set forth herein speaks only as of the date thereof.
Contacts
Jessica Morris (corporate)
Tonix Pharmaceuticals
investor.relations@tonixpharma.com
(862) 904-8182
Olipriya Das, Ph.D. (media)
Russo Partners
Olipriya.Das@russopartnersllc.com
(646) 942-5588
Peter Vozzo (investors)
ICR Westwicke
peter.vozzo@westwicke.com
(443) 213-0505
Source: Tonix Pharmaceuticals Holding Corp.
Released February 9, 2023