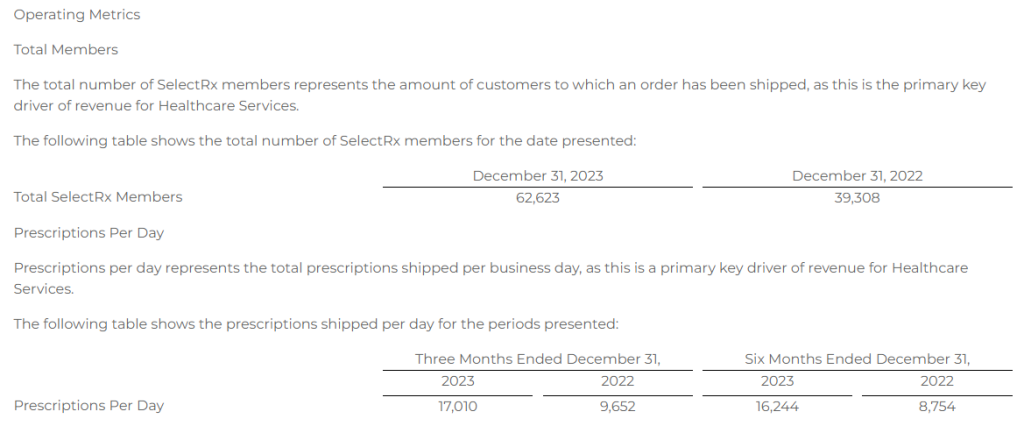
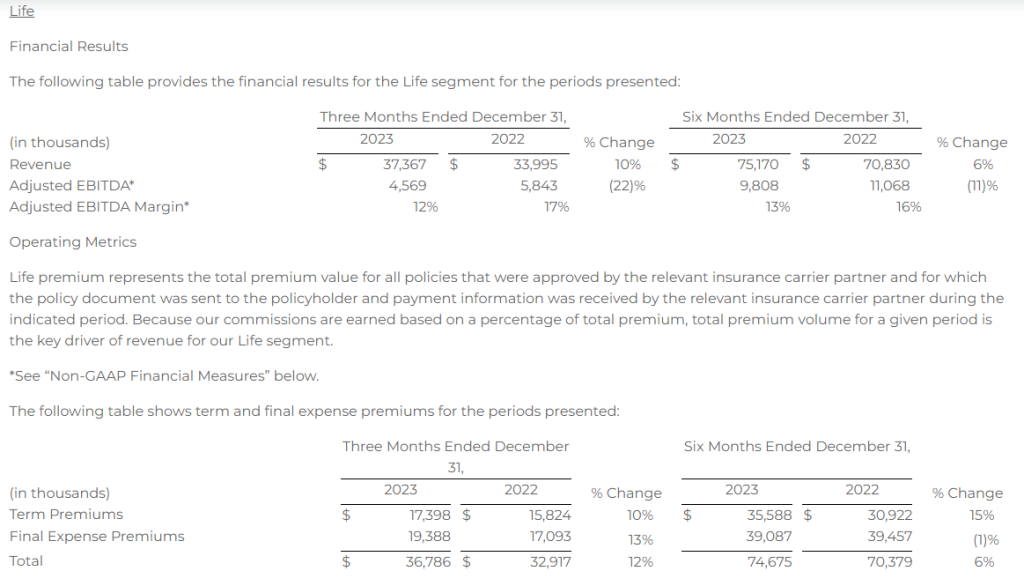
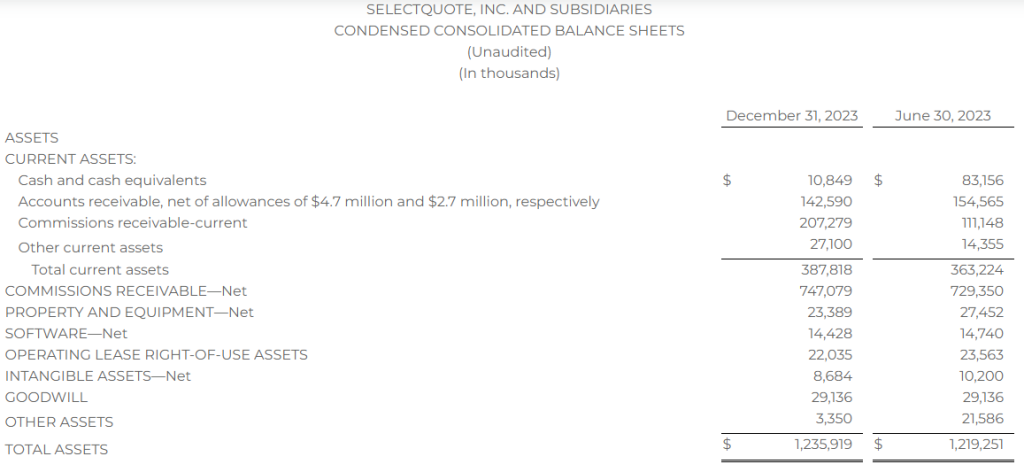
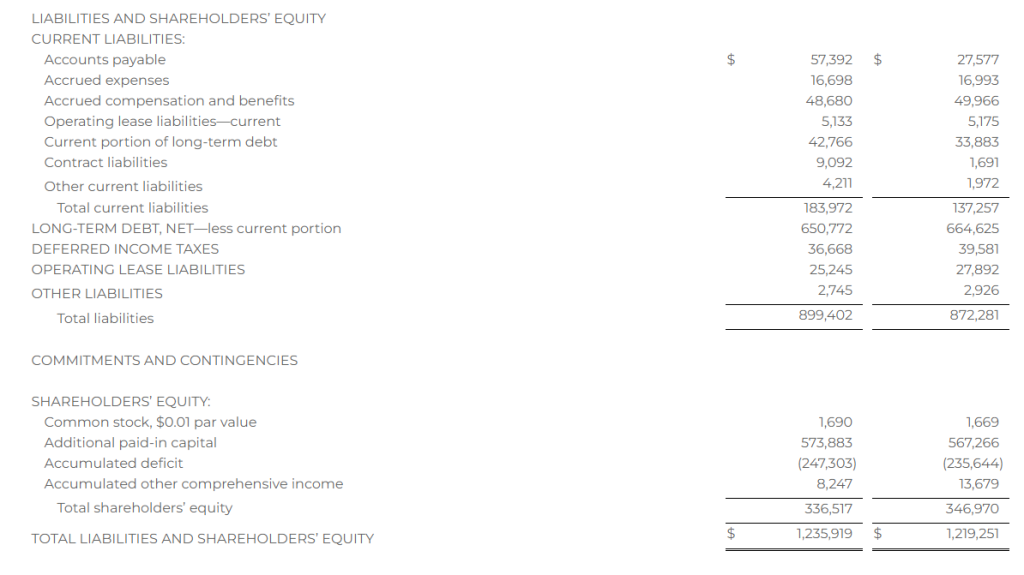
Research News and Market Data on YS
GAITHERSBURG, Md., Feb. 14, 2024 /PRNewswire/ — YS Biopharma Co., Ltd. (Nasdaq: YS) (“YS Biopharma” or the “Company”, and together with its subsidiaries, “YS Group”), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for infectious diseases and cancer, today announced the appointment of six new members to its Board of Directors (the “Board”), effective February 13, 2024. The six new directors are: Dr. Yuntao Cui; Dr. Jin Wang; Mr. Henry Chen; Mr. Haitao Zhao; Mr. Pierson Yue Pan; and Ms. Brenda Chunyuan Wu.
In addition to the new appointments, the Board has elected Dr. Ajit Shetty, who had served as the Company’s Interim Chairperson of the Board since December 9, 2023, as Chairperson of the Board, effective February 13, 2024.
Dr. Yuntao Cui has over 15 years of law-related work experience, and has previously worked as a Senior Lawyer at Zhong Lun Law Firm, one of the top law firms in China, and as General Manager of Legal Affairs at Wanda Group, a top conglomerate in the real estate, finance, and hospitality industries. Dr. Cui is currently a Senior Vice President and General Counsel at Juventas Cell Therapy Ltd., a leading immune cell therapy company. Dr. Cui holds a JD from Renmin University of China.
Dr. Jin Wang is Founding Partner and CEO of Manhattan Capital Investment Consulting Group (“MCG”), and has managed the venture capital investment fund Nuokang Venture Fund since 2014. Dr. Wang has more than 27 years of experience finding, building, and strategizing for biotech companies, including his co-founding and angel investment in leading medical professional networking and service firm DXY. Dr. Wang previously worked as an analyst and Asia/Greater China Regional Director for Paramount Capital Investment, LLC, and co-founded the Sino-American Pharmaceutical Professionals Association (“SAPA”) in 1993. Dr. Wang holds a PhD in Biomedical Science from Worcester Polytechnic Institute.
Mr. Henry Chen is the CEO of Monument Pacific Development Corp., a California-based company dedicated to developing, managing, and operating residential and commercial properties in Northern and Southern California. Mr. Chen received his MBA from the University of California, Berkeley’s Hass School of Business in 2012, and a Bachelor of Laws in Business Administration and Management from the Beijing University of Chemical Technology in 2005.
Mr. Haitao Zhao has served as a Partner of the Shanxi Tie Niu law firm since 2016, where he is responsible for handling corporate law related to intellectual property protection, corporate internal governance, and financing-related legal services. Previously, Mr. Zhao worked as an Attorney for the Beijing Ding En law firm from 2012-2016. He holds an MA in International Law from Shanxi University.
Mr. Pierson Yue Pan has served as a Vice President of Monument Pacific Development Corp. since 2018. Previously, Mr. Pan worked as Vice President of Project Development at real estate developer Propriis from 2012-2018, and as General Manager of the Africa Market with the China Civil Engineering Construction Company Nigeria Limited from 2004-2011. Mr. Pan holds an MBA from the University of California, Berkeley’s Hass School of Business.
Ms. Chunyuan (Brenda) Wu currently serves as the CFO of YS Group, a position she has held since December 31, 2020. Previously, Ms. Wu served as CFO of YS Group Biopharma from 2018-2020, and as Financial Controller for the same company from 2013-2018. Ms. Wu has also previously worked as a Senior Auditor at Ernst & Young. Ms. Wu holds degrees in Accounting and Finance from Washington State University.
Dr. Ajit Shetty, Chairperson of the Board of Directors of the Company, commented, “We are pleased to welcome each of our new directors to the Board. Each of these talented individuals brings valuable expertise in various aspects of corporate governance, legal affairs, business management, and strategic decision-making. We expect that their diverse experience and commitment to success will serve our company well going forward.”
About YS Biopharma
YS Biopharma is a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and commercializing new generations of vaccines and therapeutic biologics for infectious diseases and cancer. It has developed a proprietary PIKA® immunomodulating technology platform and a series of preventive and therapeutic biologics with a potential for improved Rabies, Coronavirus, Hepatitis B, Influenza, and Shingles vaccines. YS Biopharma operates in China, the United States, Singapore and the Philippines, and is led by a management team that combines rich local expertise and global experience in the bio-pharmaceutical industry. For more information, please visit investor.ysbiopharm.com.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, Section 21E of the Securities Exchange Act of 1934, as amended, and the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical or current fact included in this press release are forward-looking statements, including but not limited to statements regarding the expected growth of the Company, the development progress of all product candidates, the progress and results of all clinical trials, the Company’s ability to source and retain talent, and the cash position of the Company following the closing of the Business Combination. Forward-looking statements may be identified by the use of words such as “estimate,” “plan,” “project,” “forecast,” “intend,” “will,” “expect,” “anticipate,” “believe,” “seek,” “target” or other similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These statements are based on various assumptions, whether identified in this press release, and on the current expectations of YS Biopharma’s management and are not predictions of actual performance.
These statements involve risks, uncertainties and other factors that may cause actual results, levels of activity, performance, or achievements to be materially different from those expressed or implied by these forward-looking statements. Although YS Biopharma believes that it has a reasonable basis for each forward-looking statement contained in this press release, YS Biopharma cautions you that these statements are based on a combination of facts and factors currently known and projections of the future, which are inherently uncertain. In addition, there are risks and uncertainties described in the documents filed by YS Biopharma from time to time with the U.S. Securities and Exchange Commission (“SEC”). These filings may identify and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward-looking statements.
YS Biopharma cannot assure you that the forward-looking statements in this press release will prove to be accurate. These forward-looking statements are subject to a number of risks and uncertainties, including, among others, the outcome of any potential litigation, government or regulatory proceedings, the sales performance of the marketed vaccine product and the clinical trial development results of the product candidates of YS Biopharma, and other risks and uncertainties, including those included under the heading “Risk Factors” in the post-effective amendment No. 2 to Form F-1 filed with the SEC on January 23, 2024 which became effective on January 25, 2024, and other filings with the SEC. There may be additional risks that YS Biopharma does not presently know or that YS Biopharma currently believes are immaterial that could also cause actual results to differ from those contained in the forward-looking statements. In light of the significant uncertainties in these forward-looking statements, nothing in this press release should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. The forward-looking statements in this press release represent the views of YS Biopharma as of the date of this press release. Subsequent events and developments may cause those views to change. However, while YS Biopharma may update these forward-looking statements in the future, there is no current intention to do so, except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing the views of YS Biopharma as of any date subsequent to the date of this press release. Except as may be required by law, YS Biopharma does not undertake any duty to update these forward-looking statements.
Investor Relations Contact
Robin Yang
Partner, ICR, LLC
Tel: +1 (212) 537-4035
Email: YSBiopharma.IR@icrinc.com
View original content to download multimedia:https://www.prnewswire.com/news-releases/ys-biopharma-announces-appointment-of-new-directors-302061795.html
SOURCE YS Biopharma Co., Ltd.