Research News and Market Data on COCP
August 14, 2025
- Favorable CDI-988 Phase 1 safety and tolerability reported
- Challenge study with CDI-988 as a norovirus preventive and treatment planned later this year
BOTHELL, Wash., Aug. 14, 2025 (GLOBE NEWSWIRE) — Cocrystal Pharma, Inc. (Nasdaq: COCP) (“Cocrystal” or the “Company”) reports financial results for the three and six months ended June 30, 2025, and provides updates on its antiviral product pipeline, upcoming milestones and business activities.
“Preparations are underway for a Phase 1b norovirus challenge study to evaluate our potent, oral antiviral candidate CDI-988 as a prophylaxis and treatment,” said Sam Lee, Ph.D., Cocrystal’s President and co-CEO. “We are encouraged by the favorable safety and tolerability results from our Phase 1 study with CDI-988. This novel protease inhibitor has the potential to transform how we manage this highly contagious virus, which spreads rapidly in military facilities, cruise ships, nursing homes, hospitals and other confined environments. As a prophylactic treatment, CDI-988 could potentially prevent rapid spread of norovirus outbreaks in close quarters.
“We developed CDI-988 for the treatment of norovirus and coronavirus infections using our proprietary structure-based drug discovery platform technology. We are encouraged by our recent in vitro data demonstrating CDI-988 inhibits newly re-emerging norovirus GII.17 strains that are responsible for the 2024-2025 norovirus outbreaks,” added Dr. Lee.
“The absence of any approved norovirus treatments or vaccines creates a substantial market opportunity for Cocrystal,” said James Martin, Cocrystal’s CFO and co-CEO. “With 685 million global cases annually and a $60 billion worldwide economic impact, norovirus represents one of healthcare’s most pressing unmet needs.”
Antiviral Product Pipeline Overview
We harness our revolutionary, structure-based drug discovery platform technology to engineer next-generation, broad-spectrum antivirals that precisely disrupt viral replication mechanisms. Unlike traditional approaches, our technology identifies compounds that bind to highly conserved regions of viral enzymes, thereby creating a formidable defense against current viral threats as well as their mutations. By specifically targeting these evolutionary-constrained viral regions, our drug candidates maintain efficacy even as viruses mutate, while simultaneously minimizing off-target interactions that typically lead to adverse side effects. This dual advantage represents a significant breakthrough in antiviral drug development. In addition, our innovative methodology fundamentally transforms the conventional drug discovery paradigm by eliminating the inefficient, resource-intensive cycles of high-throughput compound screening and prolonged hit-to-lead optimization. The result is faster identification of promising candidates with superior resistance profiles and safety characteristics.
Influenza Programs
Influenza is a major global health threat that may become more challenging to treat due to the emergence of highly pathogenic avian influenza viruses and resistance to approved influenza antivirals. Currently approved antiviral treatments for influenza are effective but are burdened with significant viral resistance.
Each year there are approximately 1 billion cases of seasonal influenza worldwide, 3-5 million severe illnesses and up to 650,000 deaths. About 8 percent of the U.S. population gets sick from flu each season. In addition to the health risk, influenza is responsible for an estimated $10.4 billion in direct medical costs in the U.S. each year.
- Oral CC-42344 for the treatment of pandemic and seasonal influenza A
- Our novel PB2 inhibitor CC-42344 showed excellent in vitro activity against pandemic and seasonal influenza A strains, as well as strains that are resistant to Tamiflu® and Xofluza®.
- In December 2022 we reported favorable safety and tolerability results from the CC-42344 Phase 1 study.
- In December 2023 we began a randomized, double-blind, placebo-controlled Phase 2a human challenge study to evaluate the safety, tolerability, viral and clinical measurements of CC-42344 in influenza A-infected subjects in the United Kingdom, following authorization from the UK Medicines and Healthcare Products Regulatory Agency (MHRA).
- In May 2024 we completed enrollment in the Phase 2a human challenge study.
- In June 2024 we reported that in vitro studies demonstrated CC-42344 inhibits the activity of the highly pathogenic avian influenza A (H5N1) PB2 protein identified in humans exposed to infected dairy cows.
- In December 2024 we announced a plan to extend the CC-42344 Phase 2a human challenge study due to unexpectedly low influenza infection among study participants.
- In May 2025 we reported that CC-42344 was shown to be active against the highly pathogenic 2024 Texas H5N1 avian influenza strain.
- Inhaled CC-42344 as prophylaxis and treatment for pandemic and seasonal influenza A
- Our preclinical testing showed superior pulmonary pharmacology with CC-42344, including high exposure to drug and a long half-life.
- Dry powder inhalation formulation development and toxicology studies have been completed.
- Influenza A/B program
- Our efforts to develop a preclinical lead of novel influenza replication inhibitors for pandemic and season influenza are ongoing.
Norovirus Program
Norovirus is a common and highly contagious virus that afflicts people of all ages and causes symptoms of acute gastroenteritis including nausea, vomiting, stomach pain and diarrhea, as well as fatigue, fever and dehydration. There is currently no effective treatment or effective vaccine for norovirus, and the ability to curtail outbreaks is limited.
In the U.S., noroviruses are responsible for an estimated 21 million infections annually, including 109,000 hospitalizations, 465,000 emergency department visits and an estimated 900 deaths. The annual burden of norovirus to the U.S. is estimated at $10.6 billion. Noroviruses are responsible for up to 1.1 million hospitalizations and 218,000 deaths annually in children in the developing world.
- Oral pan-viral protease inhibitor CDI-988 for the treatment of noroviruses and coronaviruses
- Our novel, broad-spectrum protease inhibitor CDI-988 is being evaluated as a potential treatment for noroviruses and coronaviruses.
- CDI-988 has shown in vitro pan-viral activity against multiple norovirus strains.
- In May 2023 we announced approval of our application to the Australian regulatory agency for a randomized, double-blind, placebo-controlled Phase 1 study to evaluate the safety, tolerability and pharmacokinetics (PK) of CDI-988 in healthy subjects.
- In August 2023 we announced our selection of CDI-988 as our lead compound for the treatment for noroviruses, in addition to coronaviruses.
- In July 2024 we reported favorable safety and tolerability results from the single-ascending dose cohorts in the Phase 1 study.
- In December 2024 we reported favorable safety and tolerability results from the multiple-ascending dose cohorts of the Phase 1 study and the addition of a high-dose cohort.
- In April 2025 we announced that CDI-988 showed superior broad-spectrum antiviral activity against GII.17 strains, the most prevalent strain in the U.S. and Europe in 2024-2025.
- In August 2025 we presented favorable safety and tolerability Phase 1 data from all CDI-988 doses, including the high-dose 1200 mg cohort, at the 2025 Military Health System Research Symposium (MHSRS).
- We plan to initiate a human challenge Phase 1b study in the U.S. in 2025 to evaluate CDI-988 as a norovirus prophylaxis and treatment.
SARS-CoV-2 and Other Coronavirus Program
By targeting viral replication enzymes and proteases, we believe it is possible to develop effective treatments for all diseases caused by coronaviruses including SARS-CoV-2 and its variants, Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS). CDI-988 showed potent in vitro pan-viral activity against common human coronaviruses, rhinoviruses and respiratory enteroviruses, as well as against noroviruses. The global COVID-19 therapeutics market is estimated to exceed $16 billion annually by the end of 2031.
- Oral pan-viral protease inhibitor CDI-988 for the treatment of coronaviruses and noroviruses
- CDI-988 exhibited superior in vitro potency against SARS-CoV-2 and demonstrated a favorable safety profile and PK properties.
- In September 2023 we dosed the first healthy subject in our norovirus/coronavirus CDI-988 study, which is expected to serve as a Phase 1 study for both indications.
- In July 2024 we reported favorable safety and tolerability results from the single-ascending dose cohorts in the Phase 1 study.
- In December 2024 we reported favorable safety and tolerability results from the multiple-ascending dose cohorts of the Phase 1 study and the addition of a high-dose cohort.
- In August 2025 we presented favorable safety and tolerability Phase 1 data from all CDI-988 doses, including the high-dose 1200 mg cohort, at the MHSRS.
Second Quarter Financial Results
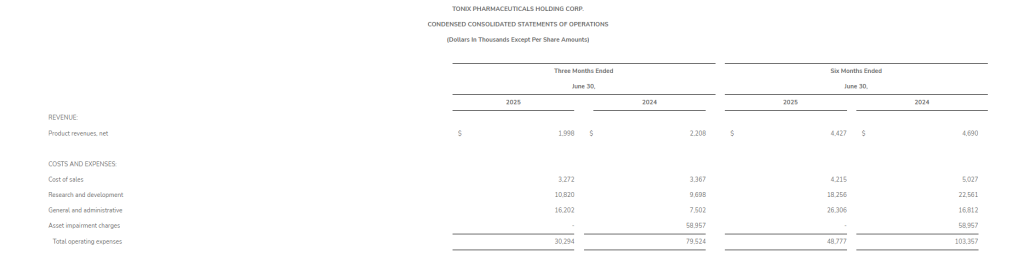
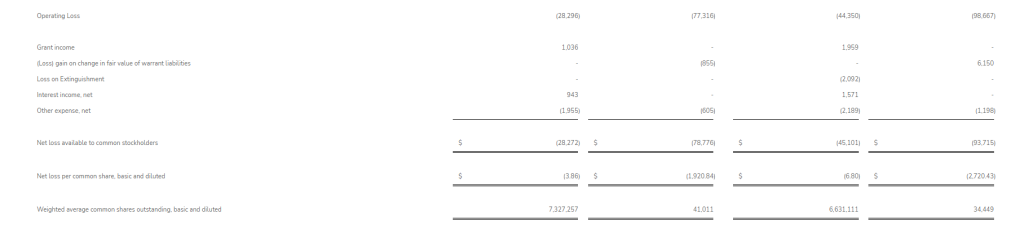
Research and development (R&D) expenses for the second quarter of 2025 were $1.1 million, compared with $4.3 million for the second quarter of 2024, with the decrease primarily due to the timing of clinical study costs. General and administrative (G&A) expenses for the second quarter of 2025 were $1.0 million, compared with $1.1 million for the second quarter of 2024, with the decrease primarily due to a reduction in salaries and wages.
Net loss for the second quarter of 2025 was $2.1 million, or $0.20 per share, compared with net loss for the second quarter of 2024 of $5.3 million, or $0.53 per share.
Six Months Financial Results
R&D expenses for the first six months of 2025 were $2.5 million, compared with $7.3 million for the first six months of 2024. G&A expenses for the first half of 2025 were $2.0 million, compared with $2.3 million for the first half of 2024.
Net loss for the first six months of 2025 was $4.4 million, or $0.43 per share, compared with a net loss for the first six months of 2024 of $9.3 million, or $0.91 per share.
Cocrystal reported unrestricted cash as of June 30, 2025 of $4.8 million, compared with $9.9 million as of December 31, 2024. Net cash used in operating activities for the first six months of 2025 was $5.1 million, compared with $8.2 million for the first six months of 2024. The Company had working capital of $4.9 million and 10.2 million common shares outstanding as of June 30, 2025.
About Cocrystal Pharma, Inc.
Cocrystal Pharma, Inc. is a clinical-stage biotechnology company discovering and developing novel antiviral therapeutics that target the replication process of influenza viruses, coronaviruses (including SARS-CoV-2), noroviruses and hepatitis C viruses. Cocrystal employs unique structure-based technologies and Nobel Prize-winning expertise to create viable antiviral drugs. For further information about Cocrystal, please visit www.cocrystalpharma.com.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements regarding our plans for the future development of preclinical and clinical product candidates including the potential of our norovirus product candidate, our plans to initiate a human Phase 1b challenge study for our norovirus product candidate, and our plans with regard to initiating a second human challenge study for CC-42344. The words “believe,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “could,” “target,” “potential,” “is likely,” “will,” “expect” and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events. Some or all of the events anticipated by these forward-looking statements may not occur. Important factors that could cause actual results to differ from those in the forward-looking statements include, but are not limited to, the risks and uncertainties arising from our need for additional capital to fund our operations over the next 12 months, inflation, the possibility of a recession, interest rate increases, imposed and threated tariffs, and geopolitical conflicts including those in Ukraine and Israel on our Company, our collaboration partners, and on the U.S., UK, Australia and global economies, including manufacturing and research delays arising from raw materials and labor shortages, supply chain disruptions and other business interruptions including any adverse impacts on our ability to obtain raw materials for and otherwise proceed with studies as well as similar problems with our vendors and our current and any future clinical research organization (CROs) and contract manufacturing organizations (CMOs), the progress and results of the studies including any adverse findings or delays, the ability of us and our CROs to recruit volunteers for, and to otherwise proceed with, clinical studies, our and our collaboration partners’ technology and software performing as expected, financial difficulties experienced by certain partners, the results of any current and future preclinical and clinical studies, general risks arising from clinical studies, receipt of regulatory approvals, regulatory changes and any adverse developments which may arise therefrom, potential mutations in a virus we are targeting that may result in variants that are resistant to a product candidate we develop, the potential for the development of effective treatments by competitors which could reduce or eliminate a prospective future market share commercializing any product candidates we may develop in the future, and our ability to meet our future liquidity needs. Further information on our risk factors is contained in our filings with the SEC, including the “Risk Factors” in Item 1A of our Annual Report on Form 10-K for the year ended December 31, 2024. Any forward-looking statement made by us herein speaks only as of the date on which it is made. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by law.
Investor Contact:
Alliance Advisors IR
Jody Cain
310-691-7100
jcain@allianceadvisors.com