Research News and Market Data on TNXP
March 13, 2023 7:00am EDT
Related Documents
Interim Analysis of Registration-Enabling Phase 3 Fibromyalgia Trial of TNX-102 SL Expected Second Quarter 2023; Topline Data Expected Fourth Quarter 2023
Potentially Pivotal Phase 2 Trials of TNX-1900 in Chronic Migraine and TNX-601 ER in Depression Scheduled for Interim Analyses in Fourth Quarter 2023
Potentially Pivotal Phase 2 Fibromyalgia-Type Long COVID Study Enrolling
Cash and Cash Equivalents Totaled Approximately $120.2 Million at December 31, 2022
CHATHAM, N.J., March 13, 2023 (GLOBE NEWSWIRE) — Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP) (Tonix or the Company), a clinical-stage biopharmaceutical company, today announced financial results for the fourth quarter and full year ended December 31, 2022, and provided an overview of recent operational highlights.
“Our clinical activity is at a high point in the Company’s history, and we believe it is setting the stage for a year of significant accomplishments across an expanded portfolio of novel pharmaceutical candidates designed to serve major unmet medical needs,” said Seth Lederman, M.D., Chief Executive Officer of Tonix.
“We are pleased with the progress of our current Phase 3 program in fibromyalgia, and we are looking forward to the results of a planned interim analysis due next quarter, followed by topline results in the fourth quarter of this year. If successful, we believe it will be the second and final adequate and well-controlled efficacy trial required for filing a New Drug Application (NDA) for approval by the U.S. Food and Drug Administration (FDA)”, he added. “Moreover, we believe we have satisfied all the other clinical and non-clinical requirements for an NDA.”
Dr. Lederman added, “Patients and caregivers alike report widespread dissatisfaction with the three currently approved drugs for fibromyalgia – Lyrica®, Cymbalta®, and Savella®, and generic pregabalin and duloxetine – switching back and forth between them, and too often taking off-label products, including addictive opiates. Fibromyalgia affects between six and 12 million adults in the U.S. according to the American Pain Association, and there hasn’t been a new FDA drug approval in the category in more than a dozen years.”
Dr Lederman continued, “Our recently expanded late-stage clinical programs include four potentially pivotal Phase 2 trials. Two are currently enrolling, one in Long COVID and the other in chronic migraine. The two others – one in depression and the other in cocaine intoxication – are due to start enrolling. We expect to initiate enrollment in the depression study by the end of March, followed by the cocaine intoxication study in the second quarter of this year.”
“In summary”, he concluded, “these programs, together with several others in earlier development, represent a diverse portfolio of programs with multiple opportunities for value creation in 2023 and beyond.”
Recent Highlights—Key Product Candidates*
Central Nervous System (CNS) Pipeline
TNX-102 SL (cyclobenzaprine HCl sublingual tablet): small molecule for the management of fibromyalgia (FM)
- The first 50% of participants were randomized on December 19, 2022, in the RESILIENT study, a double-blind, randomized, placebo-controlled, potentially pivotal confirmatory Phase 3 study of TNX-102 SL for the management of fibromyalgia. Results from a planned interim analysis are expected in the second quarter of 2023, with topline results expected in the fourth quarter of 2023. A positive topline outcome, together with results from the previous positive Phase 3 RELIEF study, would support submission of an NDA.
TNX-102 SL for the treatment of Fibromyalgia-Type Long COVID, also known as Post-Acute Sequelae of COVID-19 (PASC)
- Enrollment continues in the PREVAIL study, a potentially pivotal Phase 2 study of TNX-102 SL for fibromyalgia-type Long COVID.
- During a February 2023 virtual event co-hosted by BIO and Solve M.E. titled, “Long COVID: What Will it Take to Accelerate Therapeutic Progress?”, the Company presented its analysis that the majority of Long COVID patients present with a constellation of symptoms called nociplastic pain that overlap with fibromyalgia, and Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME) and that fibromyalgia-type Long COVID appears to be one of several chronic overlapping pain conditions (COPCs) that are related by sharing the neurological process called central sensitization.
TNX-1900 (intranasal potentiated oxytocin): small peptide for migraine, craniofacial pain, insulin resistance and related disorders, and obesity-associated binge eating disorder
- In February 2023, Tonix announced that enrollment began in the potentially pivotal Phase 2 PREVENTION study of TNX-1900 for the prevention of migraine headache in chronic migraineurs. The double-blind, placebo-controlled study has a target enrollment of 300 participants at approximately 25 sites across the U.S. Results from a planned interim analysis after the first 50% of enrolled patients have completed the study are expected in the fourth quarter of 2023.
- In January 2023, data from clinical and nonclinical studies were presented at the 16th Annual Headache Cooperative of the Pacific (HCOP) Winter Conference by collaborator Professor David Yeomans. The oral presentation titled, “Primary vs Secondary Sex Hormones and Migraine,” includes research sponsored by and licensed by Tonix. Preliminary results from a positron emission tomography (PET) study in humans showed that intranasal application of a radioisotope of magnesium-potentiated oxytocin is delivered to the trigeminal ganglia which have known roles in migraine headaches. In addition, preliminary results of data collected from isolated human trigeminal ganglia neurons in vitro show co-expression of oxytocin receptors and calcitonin gene-related peptide (CGRP), which are believed to represent the first observation of oxytocin receptors in human trigeminal ganglia. Furthermore, the presentation highlights data which suggest a sex difference in oxytocin potency.
- Tonix announced data from an in vitro study describing the impact of oxytocin on isolated human sensory neurons, presented by collaborator Professor David Yeomans at Neuroscience 2022, the annual meeting of the Society for Neuroscience. The poster, titled “In Vitro Impact of Oxytocin on Human Sensory Neurons,” is the first to show that oxytocin receptors are present on human sensory neurons and that inflammation drives expression of oxytocin receptors on these neurons. The results of this study are consistent with data from animal models and provide support for the use of oxytocin for the treatment of pain.
- An investigator-initiated Phase 2 study of TNX-1900 in obesity-associated binge eating disorder is expected to start enrolling in the second quarter of 2023 directed by principal investigator Professor Elizabeth Lawson at the Massachusetts General Hospital, a teaching hospital of Harvard Medical School.
TNX-601 ER (tianeptine hemioxalate extended-release tablets): a once-daily orally-administered small molecule for the treatment of major depressive disorder (MDD), Posttraumatic Stress Disorder (PTSD), and neurocognitive dysfunction associated with corticosteroid use.
- Enrollment is expected to initiate in the first quarter of 2023 in the potentially pivotal Phase 2 ‘UPLIFT’ Study for the treatment of MDD. Results from planned interim analysis after the first 50% of enrolled patients have completed the study are expected fourth quarter 2023.
- TNX-601 ER represents a novel approach to treating depression in the U.S., since the active ingredient tianeptine induces a neuroprotective and resilient phenotype in both neurons and microglia under conditions of stress. In contrast, antidepressants that are marketed in the U.S. act by modulating neurotransmitter levels or receptor binding in the synapse. The Phase 2 UPLIFT study is a double-blind, randomized, multicenter, placebo-controlled study to evaluate the efficacy and safety of TNX-601 ER taken orally once-daily for 6 weeks to treat MDD. It is a parallel design study with two arms, a TNX-601 ER 39.4 mg arm and a placebo arm. A total of 300 participants will be randomized in a 1:1 ratio into the two arms across approximately 30 U.S. sites, enrolling adult patients 18-65 years old with a DSM-5 diagnosis of depression and a duration for the current major depressive episode (MDE) of at least 12 weeks. The primary efficacy endpoint is mean change from baseline in the Montgomery-Åsberg Depression Rating Scale (MADRS) total score at Week 6. Key secondary efficacy endpoints include the Clinical Global Impression of Severity Scale (CGI-S) and the Sheehan Disability Scale (SDS).
TNX-1300 (recombinant double mutant cocaine esterase): biologic for life-threatening cocaine intoxication
- Tonix expects to initiate a new, potentially pivotal, Phase 2 clinical study of TNX-1300 for the treatment of cocaine intoxication in the second quarter of 2023, pending agreement with the FDA on trial design.
- As previously mentioned, in 2022, Tonix received a Cooperative Agreement grant from the National Institute on Drug Abuse (NIDA), part of the National Institutes of Health (NIH), to support development of TNX-1300.
- TNX-1300 has been granted Breakthrough Therapy designation by the FDA.
Rare Disease Pipeline
TNX-2900 (intranasal potentiated oxytocin): small peptide for the treatment of Prader-Willi syndrome (PWS)
- TNX-2900 has been granted Orphan Drug designation from the FDA for the treatment of PWS.
- As previously mentioned, in 2022, Tonix delivered a presentation titled, “TNX-2900 (Intranasal Oxytocin + Magnesium) in Development for the Treatment of Hyperphagia in Adolescents and Young Adults with Prader-Willi Syndrome” at the World Orphan Drug Congress USA.
Immunology Pipeline
TNX-1500 (anti-CD40L monoclonal antibody): third generation anti-CD40L monoclonal antibody for prophylaxis of organ transplant rejection and treatment of autoimmune disorders.
- A First-in-Human Phase 1 study is expected to start in the second quarter of 2023 of TNX-1500 for prophylaxis of organ rejection in adult patients receiving a kidney transplant.
- In February 2023, Tonix announced a research agreement with the University of Maryland, Baltimore, to study and assess the role of TNX-1500 in the prevention of heart xenograft rejection. The genetically engineered pig donors will be provided by the Revivicor Division of United Therapeutics Corporation. Preclinical xenotransplantation studies are expected to support an IND application.
- Tonix announced a research agreement with Boston Children’s Hospital to study TNX-1500 for the prevention of graft-versus-host diseases (GvHD) after hematopoietic stem cell transplantation (HCT) in animals. HCT from unrelated donors is a component of the treatment protocol for several hematologic malignancies, but GvHD complicates treatment and limits the success of engraftment after HCT.
Infectious Disease Pipeline
TNX-801 (live horsepox virus vaccine for percutaneous administration): vaccine to protect against smallpox and mpox designed as a single-administration vaccine to elicit T cell immunity
- A Phase 1 study in is expected to start in the second half of 2023.
- Tonix presented a development update from the Company’s TNX-801 vaccine program in an oral presentation at the World Vaccine and Immunotherapy Congress on December 1, 2022. The oral presentation titled, “Live Virus Smallpox and Monkeypox Vaccine,” describes the history of live virus vaccines and rationale for the development of the Company’s Recombinant Pox Virus (RPV) platform, including TNX-801 to protect against mpox and smallpox. Non-human primates vaccinated with TNX-801 were fully protected with sterilizing immunity from a lethal challenge with intra-tracheal monkeypox.
- A publication describing the activity of TNX-801 to protect non-human primates against a lethal challenge with intra-tracheal monkeypox was published in the peer-reviewed journal, Viruses (Noyce RS, et al. “Single Dose of Recombinant Chimeric Horsepox Virus (TNX-801) Vaccination Protects Macaques from Lethal Monkeypox Challenge.” Viruses. 2023 Jan 26;15(2):356. doi: 10.3390/v15020356. PMID: 36851570; PMCID: PMC9965234.)
*All of Tonix’s product candidates are investigational new drugs or biologics and none have been approved for any indication.
Recent Highlights—Corporate and Other
- In February 2023, Tonix announced the appointment of R. Newcomb Stillwell to its Board of Directors, effective March 15, 2023. Mr. Stillwell is a retired partner at Ropes & Gray LLP, an international law firm, where he devoted approximately 38 years.
- In February 2023, Tonix announced that it has exercised an option to obtain an exclusive license from Columbia University for the development of a portfolio of fully human (TNX-3600) and murine (TNX-4100) monoclonal antibodies for the treatment or prophylaxis of SARS-CoV-2 infection. The licensed monoclonal antibodies were developed as part of a research collaboration and option agreement between Tonix and Columbia University, originally announced in 2020.
- In February 2023, Tonix announced the acquisition of a preclinical portfolio of next-generation antiviral technology assets from Healion Bio, Inc. (Healion). Healion’s drug portfolio includes a class of broad-spectrum small molecule oral antiviral drug candidates including TNX-3900, formerly known as HB-121, which are cathepsin protease inhibitors, some of which have activity in vitro against SARS-CoV-2.
- On January 26, 2023, data from Tonix’s research collaboration with The University of Alberta were presented by Tom Hobman, Ph.D., Professor of Cell Biology, University of Alberta, during a presentation at the 2nd Wnt/β-catenin Targeted Drug Development Conference. The oral presentation titled, “Targeting the Wnt/β-catenin pathway as a broad-spectrum antiviral strategy,” includes research sponsored by Tonix Pharmaceuticals focused on the development and testing of Wnt/β-catenin signaling pathway inhibitors as broad-spectrum antivirals against SARS-CoV-2 and other emerging viruses.
- In January 2023, Tonix announced the publication of a paper entitled, “Development of a rapid image-based high-content imaging screening assay to evaluate therapeutic antibodies against the monkeypox virus,” in the journal Antiviral Research. The publication describes the development and optimization of two high-content image-based assays that were employed to screen for potential therapeutic antibodies against the monkeypox virus using surrogate poxviruses such as vaccinia virus. The article highlights Tonix’s TNX-3400 platform, which includes antibodies to potentially prevent or treat mpox and smallpox. These data represent the first wave of research and development conducted at the Company’s Infectious Disease R&D Center (RDC) in Frederick, Md. (Kota KP, et al., “Development of a rapid image-based high-content imaging screening assay to evaluate therapeutic antibodies against the monkeypox virus.” Antiviral Res. 2023 Feb;210:105513. doi: 10.1016/j.antiviral.2022.105513. Epub 2022 Dec 30. PMID: 36592670; PMCID: PMC9803393.)
- In January 2023, Tonix announced the appointment of Zeil Rosenberg, M.D., M.P.H. as its new Executive Vice President, Medical.
- Tonix announced data from its fully human anti-SARS-CoV-2 monoclonal antibody platform in an oral presentation at the World Antiviral Congress 2022. The presentation titled, “Platform for Generating Fully Human anti-SARS-CoV-2 Spike Therapeutic Monoclonal Antibodies” highlights the need for a broad array of monoclonal antibodies which can be scaled up quickly and potentially combined with other monoclonal antibodies to treat or prevent COVID-19. The platform is part of a broader research collaboration and option agreement with scientists at Columbia University designed to fill in important gaps in understanding the detailed immune responses to COVID-19, and to provide a foundation upon which to target vaccines and therapeutics to appropriate individuals by precision medicine.
- In December 2022, Tonix announced that it has obtained an exclusive license from Curia Global, Inc., a leading contract research, development and manufacturing organization, for the development of three humanized murine monoclonal antibodies for the treatment or prophylaxis of SARS-CoV-2 infection, the cause of COVID-19. Immunocompromised individuals, including organ transplant recipients, are at increased risk of severe COVID-19 and poor clinical outcomes. SARS-CoV-2 has mutated to evade the existing FDA Emergency Use Authorization (EUA)-approved therapeutic monoclonal antibodies.
Recent Highlights—Financial
As of December 31, 2022, Tonix had $120.2 million of cash and cash equivalents, compared to $178.7 million as of December 31, 2021. Net proceeds from financing activities were approximately $87.8 million for full year 2022, compared to $212.5 million for the full year 2021.
Since January 1, 2023, the Company repurchased 15,700,269 shares of common stock under a $12.5 million share purchase program at prices ranging from $0.44 to $1.38 for a gross aggregate cost of approximately $12.5 million.
In January 2023, the Board of Directors approved a new $12.5 million share repurchase program. Since January 1, 2023, the Company repurchased 1,000,000 shares of common stock under this share repurchase program at $1.14 for a gross aggregate cost of $1.1 million.
Cash used in operations was approximately $98.1 million for the full year 2022, compared to $75.6 million for the full year 2021. The increase in cash outlays was primarily due to an increase in research and development (R&D) and general and administrative (G&A) activities, described below.
Cash used by investing activities for the years ended December 31, 2022, and 2021 was approximately $48.1 million and $35.3 million, respectively, related to the purchase of property and equipment.
Fourth Quarter 2022 Financial Results
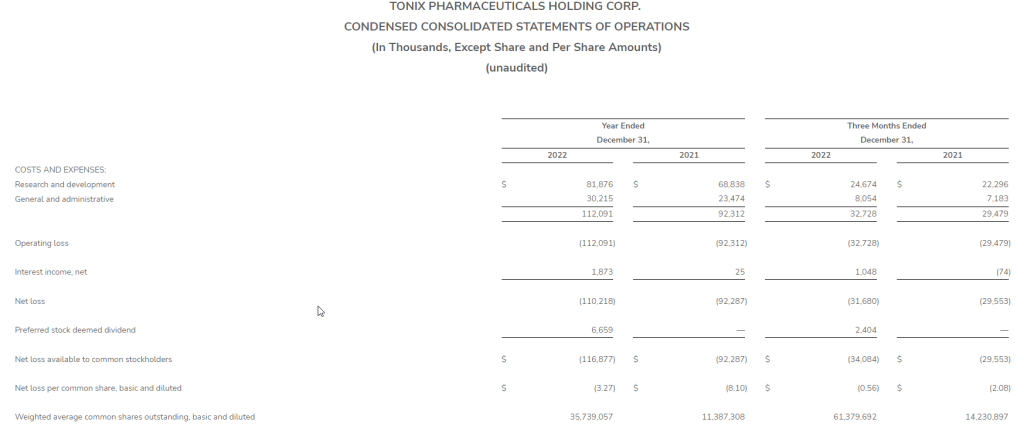
R&D expenses for the fourth quarter 2022 were $24.7 million, compared to $22.3 million for the same period in 2021. The increase is predominately due to increased employee-related, facility and laboratory expenses. We expect R&D expenses to increase during 2023 as we move our clinical development programs forward and invests in our development pipeline.
G&A expenses for the fourth quarter 2022 were $8.1 million, compared to $7.3 million for the same period in 2021. The increase is primarily due to increased employee-related and financial reporting expenses.
Net loss available to common stockholders was $34.1 million, or $0.56 per share, basic and diluted, for the fourth quarter 2022, compared to net loss of $29.6 million, or $2.08 per share, basic and diluted, for the same period in 2021. The basic and diluted weighted average common shares outstanding for the fourth quarter 2022 was 61,379,692 compared to 14,230,897 shares for the same period in 2021.
Full Year 2022 Financial Results
R&D expenses for the full year 2022 were $81.9 million, compared to $68.8 million for the same period in 2021. The increase is predominately due to increased employee-related, facility and laboratory expenses. We expect R&D expenses to increase during 2023 as we move our clinical development programs forward and invest in our development pipeline.
G&A expenses for the full year 2022 were $30.2 million, compared to $23.5 million for the same period in 2021. The increase is primarily due to increased employee-related and financial reporting expenses.
Net loss available to common stockholders was $116.9 million, or $3.27 per share, basic and diluted, for the full year 2022, compared to net loss of $92.3 million, or $8.10 per share, basic and diluted, for the same period in 2021. The basic and diluted weighted average common shares outstanding for full year 2022 was 35,739,057 compared to 11,387,308 shares for the same period in 2021.
Tonix Pharmaceuticals Holding Corp.*
Tonix is a clinical-stage biopharmaceutical company focused on discovering, licensing, acquiring and developing therapeutics to treat and prevent human disease and alleviate suffering. Tonix’s portfolio is composed of central nervous system (CNS), rare disease, immunology and infectious disease product candidates. Tonix’s CNS portfolio includes both small molecules and biologics to treat pain, neurologic, psychiatric and addiction conditions. Tonix’s lead CNS candidate, TNX-102 SL (cyclobenzaprine HCl sublingual tablet), is in mid-Phase 3 development for the management of fibromyalgia with a new Phase 3 study launched in the second quarter of 2022 and interim data expected in the second quarter of 2023. TNX-102 SL is also being developed to treat fibromyalgia-type Long COVID, a chronic post-acute COVID-19 condition. Tonix initiated a Phase 2 study in Long COVID in the third quarter of 2022. TNX-1900 (intranasal potentiated oxytocin), a small molecule in development for chronic migraine, is being studied in a potential pivotal Phase 2 study that initiated enrollment in the first quarter of 2023 and for which interim data is expected in the fourth quarter of 2023. TNX-601 ER (tianeptine hemioxalate extended-release tablets) is a once-daily formulation of tianeptine being developed as a potential treatment for major depressive disorder (MDD) with a Phase 2 study expected to be initiated in the first quarter of 2023. TNX-1300 (cocaine esterase) is a biologic designed to treat cocaine intoxication and has been granted Breakthrough Therapy designation by the FDA. A Phase 2 study of TNX-1300 is expected to be initiated in the second quarter of 2023. Tonix’s rare disease portfolio includes TNX-2900 (intranasal potentiated oxytocin) for the treatment of Prader-Willi syndrome. TNX-2900 has been granted Orphan Drug designation by the FDA. Tonix’s immunology portfolio includes biologics to address organ transplant rejection, autoimmunity and cancer, including TNX-1500, which is a humanized monoclonal antibody targeting CD40-ligand (CD40L or CD154) being developed for the prevention of allograft and xenograft rejection and for the treatment of autoimmune diseases. A Phase 1 study of TNX-1500 is expected to be initiated in the second quarter of 2023. Tonix’s infectious disease pipeline includes a vaccine in development to prevent smallpox and mpox, TNX-801; a next-generation vaccine to prevent COVID-19, TNX-1850; a platform to make fully human and murine monoclonal antibodies to treat COVID-19, TNX-3600 and TNX-4100, respectively; and humanized anti-SARS-CoV-2 monoclonal antibodies, TNX-3800; and a class of broad-spectrum small molecule oral antivirals, TNX-3900. TNX-801, Tonix’s vaccine in development to prevent smallpox and mpox, also serves as the live virus vaccine platform or recombinant pox vaccine (RPV) platform for other infectious diseases. A Phase 1 study of TNX-801 is expected to be initiated in the second half of 2023.
*All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication.
This press release and further information about Tonix can be found at www.tonixpharma.com.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking words such as “anticipate,” “believe,” “forecast,” “estimate,” “expect,” and “intend,” among others. These forward-looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, risks related to the failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID-19 pandemic and social and economic unrest; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. Tonix does not undertake an obligation to update or revise any forward-looking statement. Investors should read the risk factors set forth in the Annual Report as filed with the Securities and Exchange Commission (the “SEC”) and periodic reports filed with the SEC on or after the date thereof. All of Tonix’s forward-looking statements are expressly qualified by all such risk factors and other cautionary statements. The information set forth herein speaks only as of the date thereof.

Contacts
Jessica Morris (corporate)
Tonix Pharmaceuticals
investor.relations@tonixpharma.com
(862) 904-8182
Olipriya Das, Ph.D. (media)
Russo Partners
Olipriya.Das@russopartnersllc.com
(646) 942-5588
Peter Vozzo (investors)
ICR Westwicke
peter.vozzo@westwicke.com
(443) 213-0505