Research News and Market Data on TSQ
Digital Represents 52% of September YTD Total Net Revenue
Ignite’s Digital Advertising Revenue Growth Accelerates in Q3
Repurchased $25 Million of Debt ($36M through October) and $24 Million of Equity in September YTD Period
PURCHASE, N.Y., Nov. 07, 2024 (GLOBE NEWSWIRE) — Townsquare Media, Inc. (NYSE: TSQ) (“Townsquare”, the “Company,” “we,” “us,” or “our”) announced today its financial results for the third quarter ended September 30, 2024.
“I am pleased to share that Townsquare’s net revenue returned to year-over-year growth, driven by sequential improvement across each of our three business segments, due to our local focus and our unique and differentiated digital platform, as well as the benefit from political revenue. Third quarter net revenue increased +0.2% year-over-year and Adjusted EBITDA decreased -6.3% year-over-year, both meeting guidance and reflecting a sequential improvement from the first and second quarter. In addition, net income improved $47.8 million year-over-year, in large part due to a reduction in non-cash impairment charges,” commented Bill Wilson, Chief Executive Officer of Townsquare Media, Inc. “Our return to net revenue growth in the third quarter coincided with our return to total Digital net revenue growth, which increased by +1% year-over-year. In particular, Townsquare Interactive’s sequential revenue growth improved to +3% quarter-over-quarter, and Digital Advertising net revenue increased +5% year-over-year, an acceleration from the +1% revenue growth rates in the first six months of 2024. In total, Digital represented more than half of Townsquare’s net revenue in the first nine months of the year, a true point of differentiation from others in local media, as we have evolved from a local broadcast radio company that was founded in 2010, to a Digital First Local Media Company with a world class team and a unique and differentiated strategy, assets, platforms and solutions.”
Mr. Wilson continued, “We have executed and delivered on what we said we would do, while simultaneously building value for our shareholders through dividend payments, debt reduction and share repurchases. In the first nine months of the year, we have repurchased and retired $25 million of our bonds at a discount to par ($36 million through October), and repurchased $24 million of equity, or 2.3 million shares, including the accretive share repurchase of 1.5 million shares from Madison Square Garden. At the same time, we have maintained our high yielding dividend and a strong cash balance, which was $22 million at the end of the third quarter, and net leverage remained below 4.9x. We are gearing up for our upcoming refinancing, and we look forward to sharing that outcome with our investors when we next report.”
The Company announced today that its Board of Directors approved a quarterly cash dividend of $0.1975 per share. The dividend will be payable on February 1, 2025 to shareholders of record as of the close of business on January 21, 2025. As of yesterday’s closing price that reflects a dividend yield of approximately 8%.
Segment Reporting
We have three reportable operating segments, Subscription Digital Marketing Solutions, Digital Advertising and Broadcast Advertising. The Subscription Digital Marketing Solutions segment includes our subscription digital marketing solutions business, Townsquare Interactive. The Digital Advertising segment, marketed externally as Townsquare Ignite, includes digital advertising on our owned and operated digital properties, our first party data digital management platform and our digital programmatic advertising platform. The Broadcast Advertising segment includes our local, regional, and national advertising products and solutions delivered via terrestrial radio broadcast, and other miscellaneous revenue that is associated with our broadcast advertising platform. The remainder of our business is reported in the Other category, which includes our live events business.
Third Quarter Results*
- As compared to the third quarter of 2023:
- Net revenue increased 0.2%, and decreased 2.5% excluding political
- Net income increased $47.8 million
- Adjusted EBITDA decreased 6.3%
- Total Digital net revenue increased 1.1%
- Subscription Digital Marketing Solutions (“Townsquare Interactive”) net revenue decreased 5.8%
- Digital Advertising net revenue increased 4.7%
- Total Digital Adjusted Operating Income decreased 8.9%
- Subscription Digital Marketing Solutions Adjusted Operating Income decreased 11.0%
- Digital Advertising Adjusted Operating Income decreased 7.9%
- Broadcast Advertising net revenue increased 0.3%, and decreased 5.3% excluding political
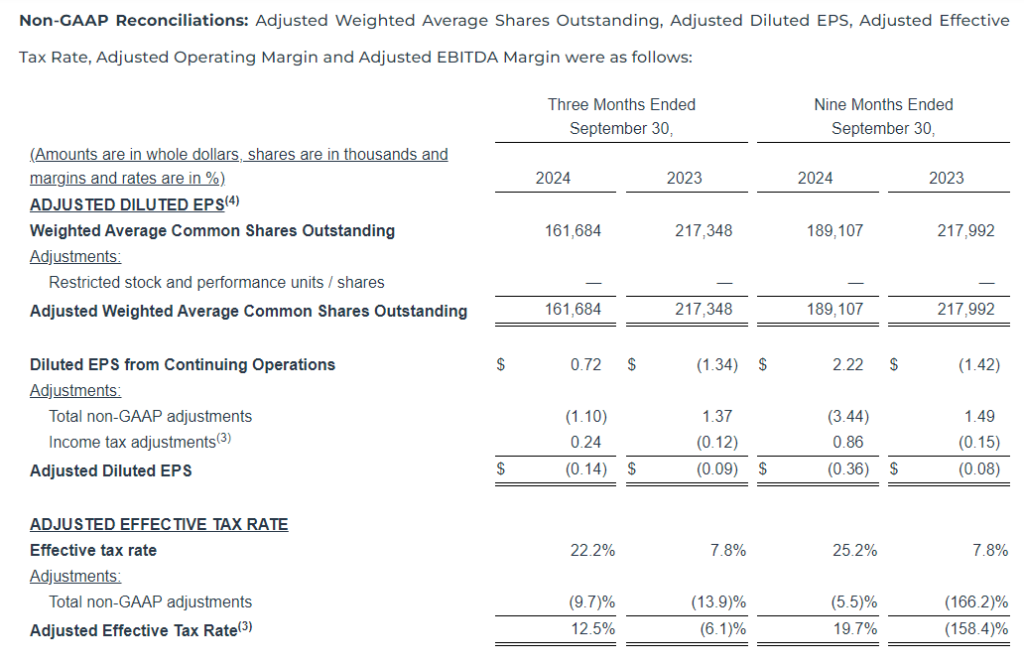
- Net Income per diluted share was $0.63 and Adjusted Net Income per diluted share was $0.35
- Repurchased an aggregate $11.0 million of our 2026 Senior Secured Notes below par
- Repurchased 0.1 million shares of the Company’s common stock at an average price of $11.32
Year-to-Date Highlights*
- As compared to the nine months ended September 30, 2023:
- Net revenue decreased 1.8%, and 3.3% excluding political
- Net loss decreased $5.2 million
- Adjusted EBITDA decreased 8.0%
- Total Digital net revenue decreased 2.6%
- Subscription Digital Marketing Solutions net revenue decreased 11.5%
- Digital Advertising net revenue increased 2.4%
- Total Digital Adjusted Operating Income decreased 17.0%
- Subscription Digital Marketing Solutions Adjusted Operating Income decreased 10.3%
- Digital Advertising Adjusted Operating Income decreased 20.2%
- Broadcast Advertising net revenue decreased 0.3%, and 3.4%, excluding political
- Repurchased an aggregate $24.7 million of our 2026 Senior Secured Notes below par
- Repurchased 2.3 million shares of the Company’s common stock at an average price of $10.31
- Repurchased and retired 3.2 million options expiring in July 2024 for a net purchase price of $3.60 per option
*See below for discussion of non-GAAP measures.
Guidance
For the fourth quarter of 2024, net revenue is expected to be between $114.8 million and $118.8 million, and Adjusted EBITDA is expected to be between $30.8 million and $31.8 million.
For the full year 2024, net revenue is expected to be between $448 million and $452 million, and Adjusted EBITDA is expected to be between $100 million and $101 million, both within our original guidance ranges.
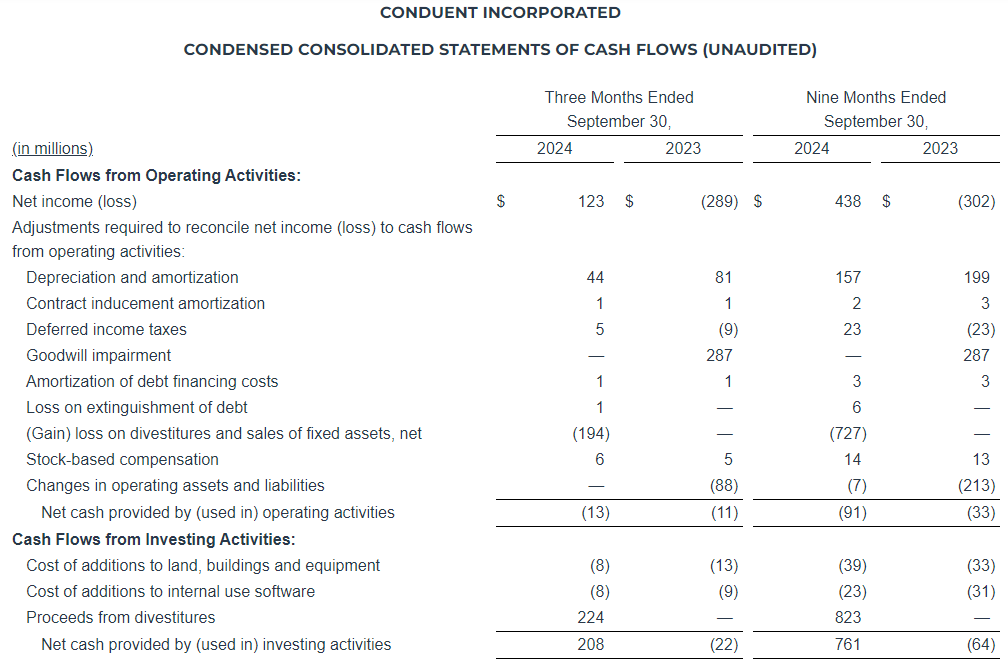
Quarter Ended September 30, 2024 Compared to the Quarter Ended September 30, 2023
Net Revenue
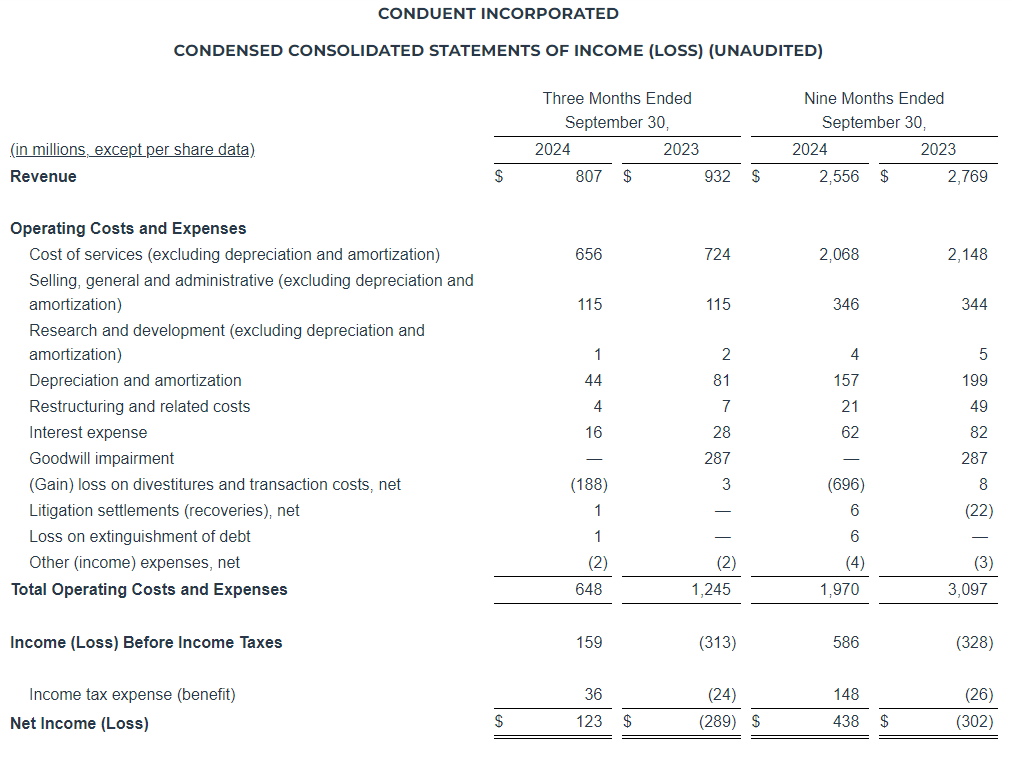
Net revenue for the three months ended September 30, 2024 increased $0.2 million, or 0.2%, to $115.3 million as compared to $115.1 million in the same period in 2023. Digital Advertising net revenue increased $1.9 million, or 4.7%, as compared to the same period in 2023, and Broadcast Advertising net revenue increased $0.2 million, or 0.3%, as compared to the same period in 2023. These increases were partially offset by a decrease in Subscription Digital Marketing Solutions net revenue of $1.2 million, or 5.8%, and a $0.6 million, or 37.3%, decrease in Other net revenue as compared to the same period in 2023. Excluding political revenue of $3.7 million and $0.6 million for the three months ended September 30, 2024 and 2023, respectively, net revenue decreased $2.9 million, or 2.5%, to $111.6 million. Broadcast Advertising net revenue decreased $2.8 million, or 5.3%, to $50.8 million, and Digital Advertising net revenue increased $1.8 million, or 4.6%, to $40.7 million.
Net Income (Loss)
For the three months ended September 30, 2024, we reported net income of $11.3 million, an increase of $47.8 million as compared to a net loss of $36.5 million in the same period last year. The increase was primarily due to a $29.0 million decrease in non-cash impairment charges, partially offset by a $2.5 million increase in direct operating expenses and a $22.6 million decrease in the income tax provision due to the valuation allowance for interest expense carryforwards and an increase in certain non-deductible compensation costs. Adjusted Net Income decreased $2.2 million as compared to the same period last year.
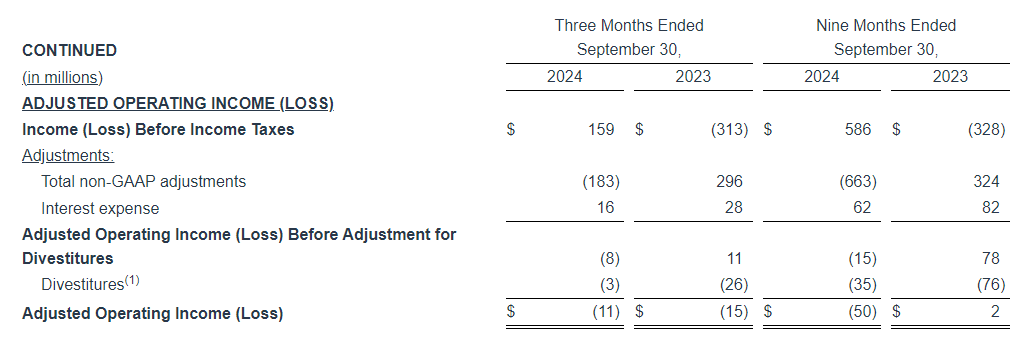
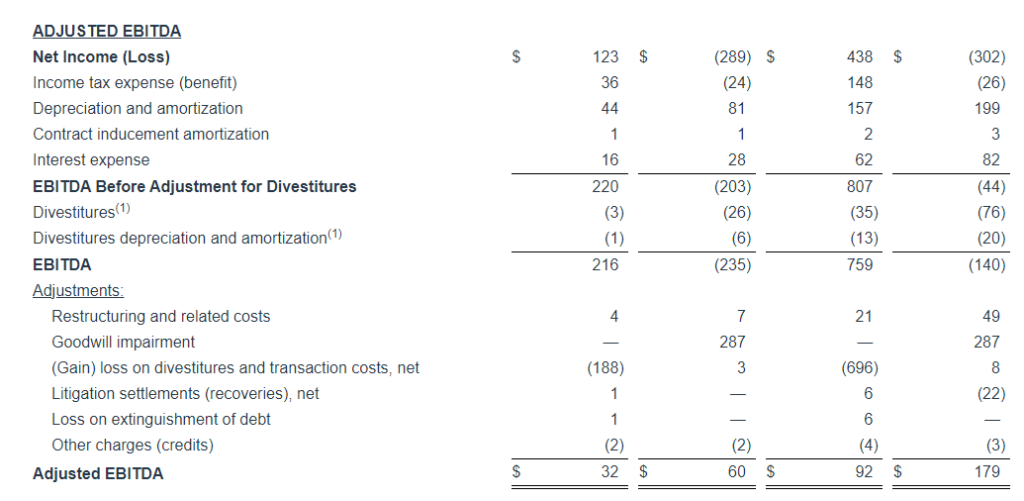
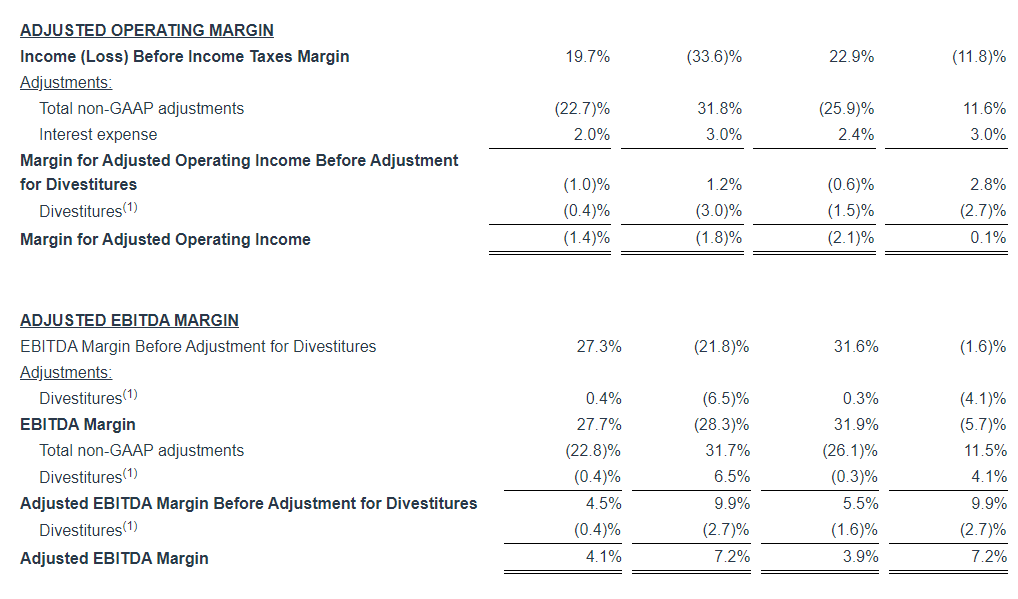
Adjusted EBITDA
Adjusted EBITDA for the three months ended September 30, 2024 decreased $1.7 million, or 6.3%, to $25.5 million, as compared to $27.2 million in the same period last year. Adjusted EBITDA (Excluding Political) decreased $4.3 million, or 16.3%, to $22.3 million, as compared to $26.6 million in the same period last year.
Nine Months Ended September 30, 2024 Compared to the Nine Months Ended September 30, 2023
Net Revenue
Net revenue for the nine months ended September 30, 2024, decreased $6.3 million, or 1.8%, to $333.2 million as compared to $339.4 million in the same period in 2023. Subscription Digital Marketing Solutions net revenue decreased $7.2 million, or 11.5%, Other net revenue decreased $1.3 million, or 15.3%, and Broadcast Advertising net revenue decreased $0.4 million, or 0.3%, as compared to the same period in 2023. These declines were partially offset by a $2.7 million, or 2.4%, increase in Digital Advertising net revenue as compared to the same period in 2023. Excluding political revenue of $6.2 million and $1.2 million for the nine months ended September 30, 2024 and 2023, respectively, net revenue decreased $11.3 million, or 3.3% to $327.0 million, Broadcast Advertising net revenue decreased $5.1 million, or 3.4%, to $147.6 million, and Digital Advertising net revenue increased $2.5 million, or 2.2%, to $116.2 million.
Net Loss
For the nine months ended September 30, 2024, we reported a net loss of $36.0 million, a decrease of $5.2 million as compared to a net loss of $41.1 million in the same period last year. The decrease was due to a $29.4 million decrease in non-cash impairment charges, partially offset by increases in stock-based compensation and transaction and business realignment costs, the decrease in net revenue and a $4.5 million increase in the income tax provision was driven by the valuation allowance for interest expense carryforwards and an increase in certain non-deductible compensation costs. Adjusted Net Income decreased $9.7 million as compared to the same period last year.
Adjusted EBITDA
Adjusted EBITDA for the nine months ended September 30, 2024 decreased $6.0 million, or 8.0% to $69.2 million, as compared to $75.2 million in the same period last year. Adjusted EBITDA (Excluding Political) decreased $10.3 million, or 13.8%, to $63.9 million, as compared to $74.2 million in the same period last year.
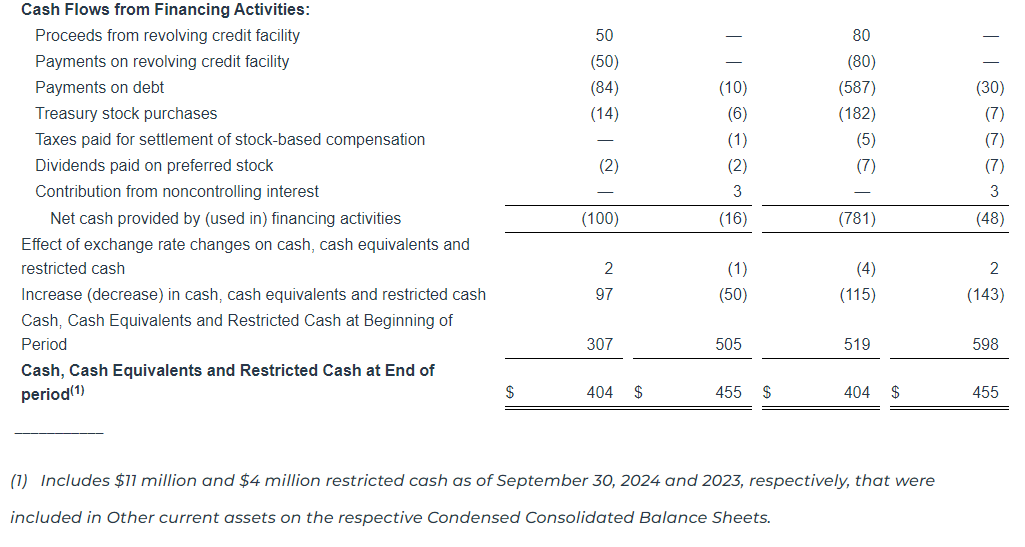
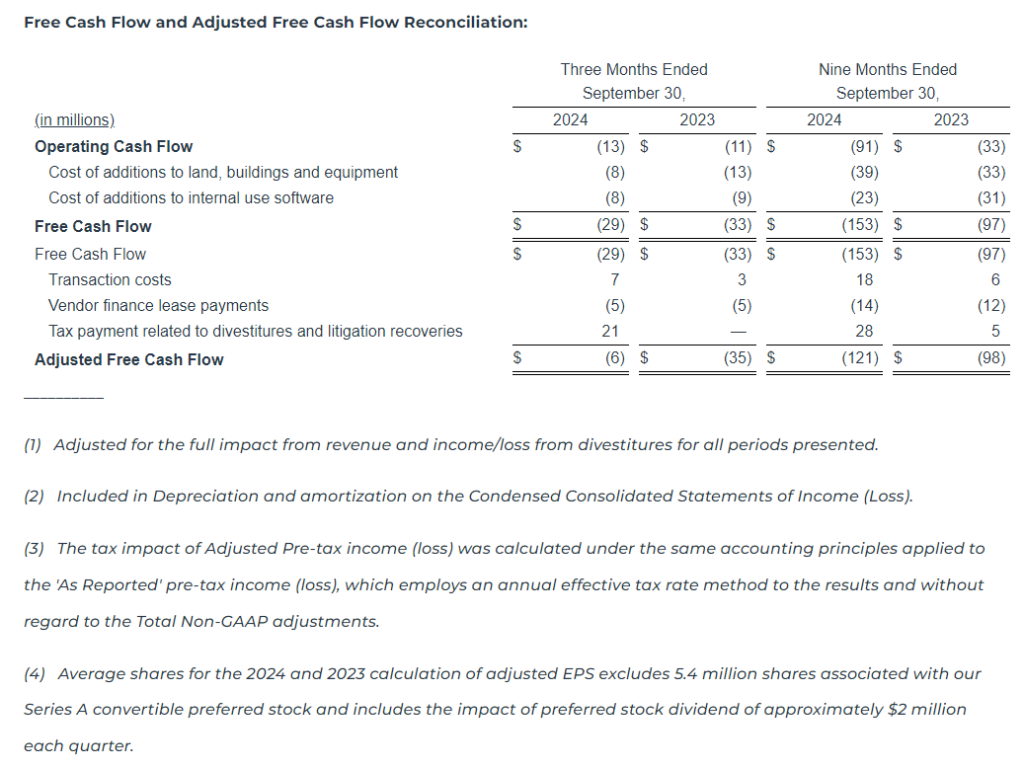
Liquidity and Capital Resources
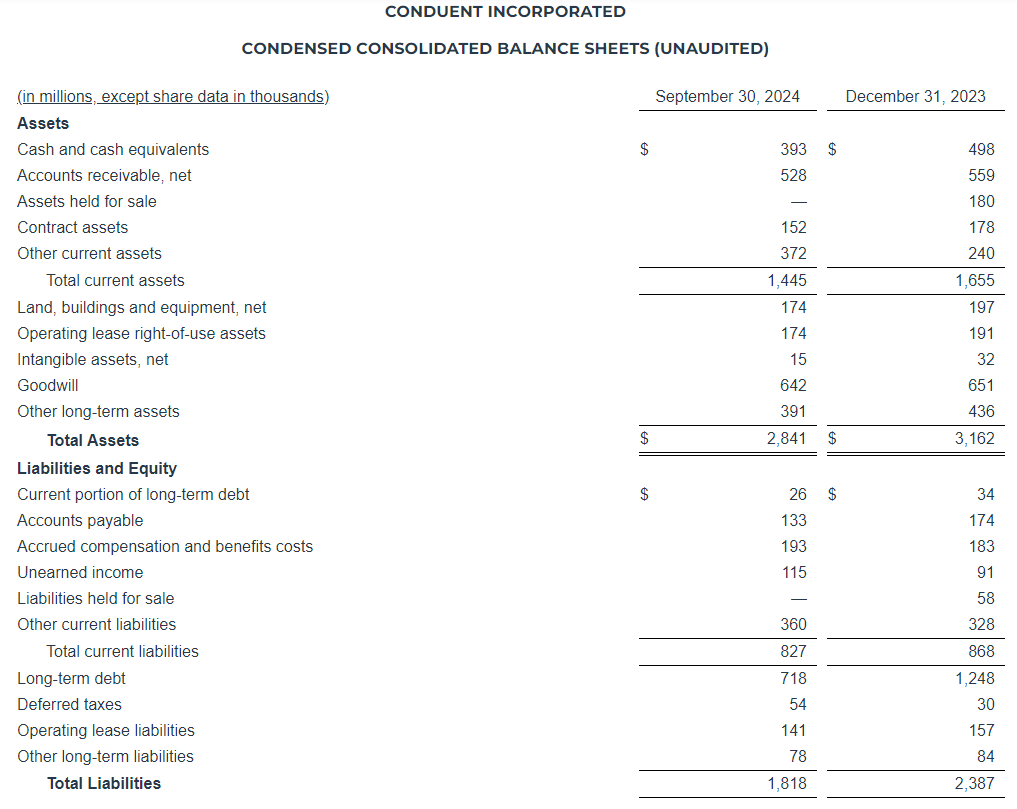
As of September 30, 2024, we had a total of $21.8 million of cash and cash equivalents and $478.9 million of outstanding indebtedness, representing 5.10x and 4.86x gross and net leverage, respectively, based on Adjusted EBITDA for the twelve months ended September 30, 2024, of $94.0 million.
The table below presents a summary, as of November 1, 2024, of our outstanding common stock (net of treasury shares).
| Security | Number Outstanding | Description | ||
| Class A common stock | 14,231,917 | One vote per share. | ||
| Class B common stock | 815,296 | 10 votes per share.1 | ||
| Class C common stock | 500,000 | No votes.1 | ||
| Total | 15,547,213 | |||
| 1 Each share converts into one share of Class A common stock upon transfer or at the option of the holder, subject to certain conditions, including compliance with FCC rules. | ||||
Conference Call
Townsquare Media, Inc. will host a conference call to discuss certain third quarter 2024 financial results and 2024 guidance on Thursday, November 7, 2024 at 10:00 a.m. Eastern Time. The conference call dial-in number is 1-800-717-1738 (U.S. & Canada) or 1-646-307-1865 (International) and the conference ID is “Townsquare”. A live webcast of the conference call will also be available on the investor relations page of the Company’s website at www.townsquaremedia.com.
A replay of the conference call will be available through November 14, 2024. To access the replay, please dial 1-844-512-2921 (U.S. and Canada) or 1-412-317-6671 (International) and enter confirmation code 1142541. A web-based archive of the conference call will also be available at the above website.
About Townsquare Media, Inc.
Townsquare is a community-focused digital media and digital marketing solutions company with market leading local radio stations, principally focused outside the top 50 markets in the U.S. Our assets include a subscription digital marketing services business, Townsquare Interactive, providing website design, creation and hosting, search engine optimization, social media and online reputation management as well as other digital monthly services for SMBs; a robust digital advertising division, Townsquare Ignite, a powerful combination of a) an owned and operated portfolio of more than 400 local news and entertainment websites and mobile apps along with a network of leading national music and entertainment brands, collecting valuable first party data and b) a proprietary digital programmatic advertising technology stack with an in-house demand and data management platform; and a portfolio of 349 local terrestrial radio stations in 74 U.S. markets strategically situated outside the Top 50 markets in the United States. Our portfolio includes local media brands such as WYRK.com, WJON.com and NJ101.5.com, and premier national music brands such as XXLmag.com, TasteofCountry.com, UltimateClassicRock.com, and Loudwire.com. For more information, please visit www.townsquaremedia.com, www.townsquareinteractive.com and www.townsquareignite.com.
Forward-Looking Statements
Except for the historical information contained in this press release, the matters addressed are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements often discuss our current expectations and projections relating to our financial condition, results of operations, plans, objectives, future performance and business. You can identify forward-looking statements by the fact that they do not relate strictly to historical or current facts. These statements may include words such as “aim,” “anticipate,” “estimate,” “expect,” “forecast,” “outlook,” “potential,” “project,” “projection,” “plan,” “intend,” “seek,” “believe,” “may,” “could,” “would,” “will,” “should,” “can,” “can have,” “likely,” the negatives thereof and other words and terms. Actual events or results may differ materially from the results anticipated in these forward-looking statements as a result of a variety of factors. While it is impossible to identify all such factors, factors that could cause actual results to differ materially from those estimated by us include the impact of general economic conditions in the United States, or in the specific markets in which we currently do business including supply chain disruptions, inflation, labor shortages and the effect on advertising activity, industry conditions, including existing competition and future competitive technologies, the popularity of radio as a broadcasting and advertising medium, cancellations, disruptions or postponements of advertising schedules in response to national or world events, our ability to develop and maintain digital technologies and hire and retain technical and sales talent, our dependence on key personnel, our capital expenditure requirements, our continued ability to identify suitable acquisition targets, and consummate and integrate any future acquisitions, legislative or regulatory requirements, risks and uncertainties relating to our leverage and changes in interest rates, our ability to obtain financing at times, in amounts and at rates considered appropriate by us, our ability to access the capital markets as and when needed and on terms that we consider favorable to us and other factors discussed in this section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this report and under “Risk Factors” in our 2023 Annual Report on Form 10-K, for the year ended December 31, 2023, filed with the SEC on March 15, 2024, as well as other risks discussed from time to time in our filings with the SEC. Many of these factors are beyond our ability to predict or control. In addition, as a result of these and other factors, our past financial performance should not be relied on as an indication of future performance. The cautionary statements referred to in this section also should be considered in connection with any subsequent written or oral forward-looking statements that may be issued by us or persons acting on our behalf. The forward-looking statements included in this report are made only as of the date hereof or as of the date specified herein. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Non-GAAP Financial Measures and Definitions
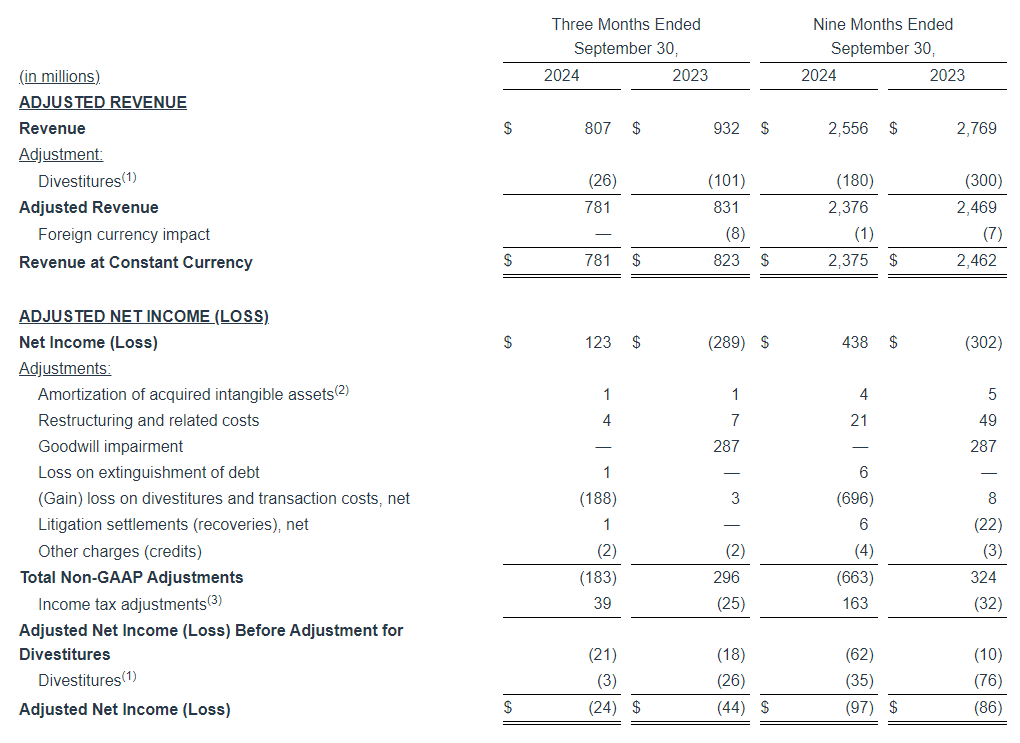
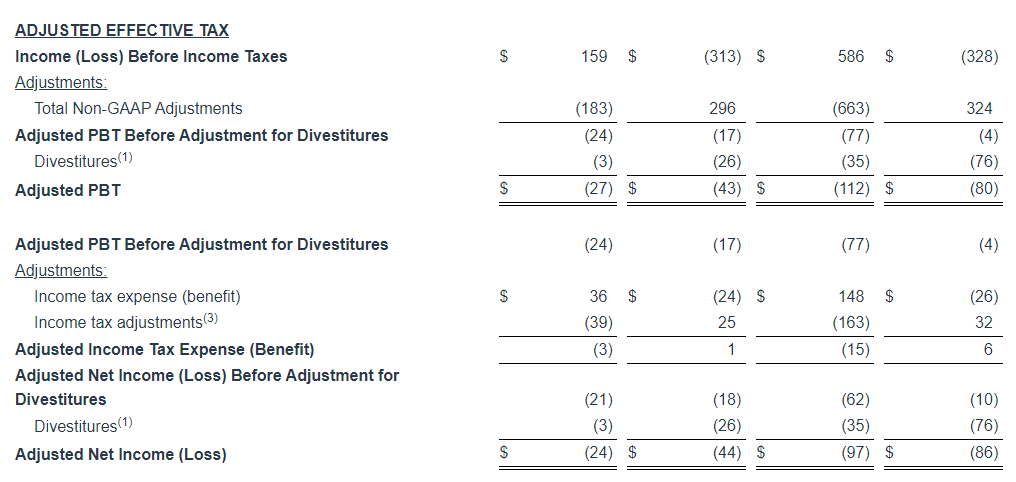
In this press release, we refer to Adjusted Operating Income, Adjusted EBITDA, Adjusted EBITDA (Excluding Political), Adjusted Net Income and Adjusted Net Income Per Share which are financial measures that have not been prepared in accordance with generally accepted accounting principles in the United States (“GAAP”).
We define Adjusted Operating Income by Segment as operating income by segment before the deduction of depreciation and amortization, stock-based compensation, corporate expenses, transaction costs, business realignment costs, impairments and net loss (gain) on sale and retirement of assets. We define Adjusted EBITDA as net income before the deduction of income taxes, interest expense, net, gain on repurchases of debt, transaction and business realignment costs, depreciation and amortization, stock-based compensation, impairments, net loss (gain) on sale and retirement of assets and other expense (income) net. We define Adjusted EBITDA (Excluding Political) as Adjusted EBITDA less political net revenue, net of a fifteen percent deduction to account for estimated national representative firm fees, music licensing fees and sales commissions expense. Adjusted Net Income is defined as net income before the deduction of transaction and business realignment costs, impairments, gains on sale of investments, change in fair value of investment, net loss (gain) on sale and retirement of assets, gain on repurchases of debt, gain on sale of digital assets, gain on insurance recoveries and net income attributable to non-controlling interest, net of income taxes stated at the Company’s applicable statutory effective tax rate. Adjusted Net Income Per Share is defined as Adjusted Net Income divided by the weighted average shares outstanding. We define Net Leverage as our total outstanding indebtedness, net of our total cash balance as of September 30, 2024, divided by our Adjusted EBITDA for the twelve months ended September 30, 2024. These measures do not represent, and should not be considered as alternatives to or superior to, financial results and measures determined or calculated in accordance with GAAP. In addition, these non-GAAP measures are not based on any comprehensive set of accounting rules or principles. You should be aware that in the future we may incur expenses or charges that are the same as or similar to some of the adjustments in the presentation, and we do not infer that our future results will be unaffected by unusual or non-recurring items. In addition, these non-GAAP measures may not be comparable to similarly-named measures reported by other companies.
We use Adjusted Operating Income by Segment to evaluate the operating performance of our business segments. We use Adjusted EBITDA and Adjusted EBITDA (Excluding Political) to facilitate company-to-company operating performance comparisons by backing out potential differences caused by variations in capital structures (affecting interest expense), taxation and the age and book depreciation of facilities and equipment (affecting relative depreciation expense), which may vary for different companies for reasons unrelated to operating performance, and to facilitate year over year comparisons, by backing out the impact of political revenue which varies depending on the election cycle and may be unrelated to operating performance. We use Adjusted Net Income and Adjusted Net Income Per Share to assess total company operating performance on a consistent basis. We use Net Leverage to measure the Company’s ability to handle its debt burden. We believe that these measures, when considered together with our GAAP financial results, provide management and investors with a more complete understanding of our business operating results, including underlying trends, by excluding the effects of transaction costs, net loss (gain) on sale and retirement of assets, business realignment costs and certain impairments. Further, while discretionary bonuses for members of management are not determined with reference to specific targets, our board of directors may consider Adjusted Operating Income by Segment, Adjusted EBITDA, Adjusted EBITDA (Excluding Political), Adjusted Net Income, Adjusted Net Income Per Share, and Net Leverage when determining discretionary bonuses.
Investor Relations
Claire Yenicay
(203) 900-5555
investors@townsquaremedia.com