Research News and Market Data on LWAY
13 Nov, 2023, 09:00 ET
Net sales increase 7.2% year-over-year to $40.9 million; 16th consecutive quarter of year-over-year net sales growth
730 basis points of year-over-year gross profit margin expansion
MORTON GROVE, Ill., Nov. 13, 2023 /PRNewswire/ — Lifeway Foods, Inc. (Nasdaq: LWAY) (“Lifeway” or “the Company”), a leading U.S. supplier of kefir and fermented probiotic products to support the microbiome, today reported financial results for the third quarter ended September 30, 2023.
“I am excited to announce that our strong momentum continued in the third quarter as we once again set a Company record on the topline, and delivered robust, year-over-year gross profit margin expansion of 730 basis points,” commented Julie Smolyansky, President and Chief Executive Officer of Lifeway Foods. “Net sales were up 7.2%, marking our 16th consecutive quarter of year-over-year growth, and continued to be driven by volume growth in our flagship Lifeway drinkable kefir. This growth is particularly impressive as we lapped an exceptional third quarter of 2022, illustrating both the loyalty of our customers, who have maintained their dedication to our premium, healthy offerings in light of inflation-justified price increases last year, as well as the success of our strategic investments in capturing incremental consumers seeking better-for-you offerings at a great value. Additionally, our proactive operating discipline and favorable milk pricing helped achieve vastly improved year-over-year profitability alongside the heightened sales, a testament to the execution by the whole Lifeway team. Looking ahead, we will continue to assess further distribution opportunities and pursue additional brand exposure for our core Lifeway kefir products and farmer cheese. This was an amazing start to the second half of 2023, and I want to thank the Lifeway team, our customers and retail partners for helping us deliver yet another quarter of record revenue.”
Third Quarter 2023 Results
Net sales were $40.9 million for the third quarter ended September 30, 2023, an increase of $2.8 million or 7.2% from the same period of 2022. The net sales increase was primarily driven by higher volumes of Lifeway branded drinkable kefir, and to a lesser extent the impact of price increases implemented during the fourth quarter of 2022.
Gross profit as a percentage of net sales was 27.2% for the third quarter ended September 30, 2023, compared to 19.9% in the same period of 2022. The 730 basis point increase versus the prior year was primarily due to the higher volumes of Lifeway branded products and the favorable impact of milk pricing, and to a lesser extent the price increases implemented during the fourth quarter of 2022 and decreased transportation costs.
Selling, general and administrative expenses as a percentage of net sales were 14.6% for the third quarter ended September 30, 2023, compared to 16.4% in the same period of 2022.
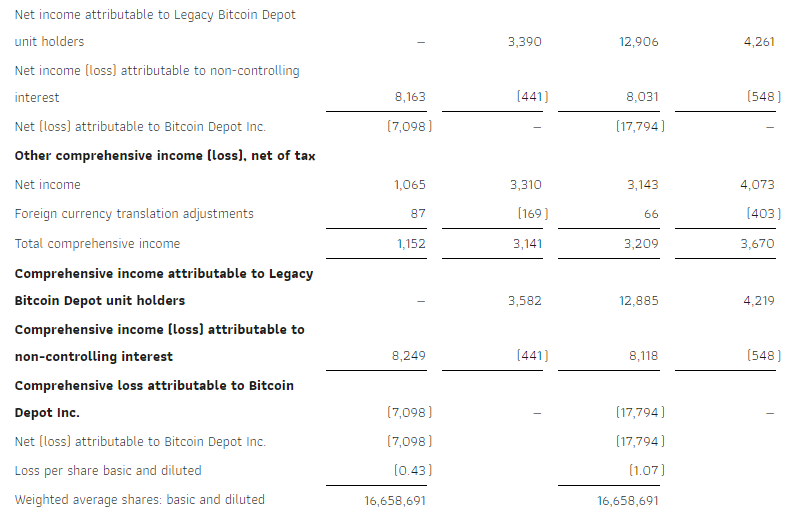
The Company reported net income of $3.4 million or $0.23 per basic and diluted common share for the third quarter ended September 30, 2023 compared to net income of $1.0 million or $0.06 per basic and diluted common share during the same period in 2022.
Conference Call and Webcast
A pre-recorded conference call and webcast with Julie Smolyansky discussing these results with additional comments and details is available through the “Investor Relations” section of the Company’s website at https://lifewaykefir.com/webinars-reports/ and will also be available for replay.
About Lifeway Foods, Inc.
Lifeway Foods, Inc., which has been recognized as one of Forbes’ Best Small Companies, is America’s leading supplier of the probiotic, fermented beverage known as kefir. In addition to its line of drinkable kefir, the company also produces cheese, probiotic oat milk, and a ProBugs line for kids. Lifeway’s tart and tangy fermented dairy products are now sold across the United States, Mexico, Ireland and France. Learn how Lifeway is good for more than just you at lifewayfoods.com.
Forward-Looking Statements
This release (and oral statements made regarding the subjects of this release) contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, future operating and financial performance, product development, market position, business strategy and objectives. These statements use words, and variations of words, such as “continue,” “build,” “future,” “increase,” “drive,” “believe,” “look,” “ahead,” “confident,” “deliver,” “outlook,” “expect,” and “predict.” Other examples of forward-looking statements may include, but are not limited to, (i) statements of Company plans and objectives, including the introduction of new products, or estimates or predictions of actions by customers or suppliers, (ii) statements of future economic performance, and (III) statements of assumptions underlying other statements and statements about Lifeway or its business. You are cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events and thus are inherently subject to uncertainty. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from Lifeway’s expectations and projections. These risks, uncertainties, and other factors include: price competition; the decisions of customers or consumers; the actions of competitors; changes in the pricing of commodities; the effects of government regulation; possible delays in the introduction of new products; and customer acceptance of products and services. A further list and description of these risks, uncertainties, and other factors can be found in Lifeway’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022, and the Company’s subsequent filings with the SEC. Copies of these filings are available online at https://www.sec.gov, http://lifewaykefir.com/investor-relations/, or on request from Lifeway. Information in this release is as of the dates and time periods indicated herein, and Lifeway does not undertake to update any of the information contained in these materials, except as required by law. Accordingly, YOU SHOULD NOT RELY ON THE ACCURACY OF ANY OF THE STATEMENTS OR OTHER INFORMATION CONTAINED IN ANY ARCHIVED PRESS RELEASE.
Media:
Derek Miller
Vice President of Communications, Lifeway Foods
Email: derekm@lifeway.net
General inquiries:
Lifeway Foods, Inc.
Phone: 847-967-1010
Email: info@lifeway.net
| LIFEWAY FOODS, INC. AND SUBSIDIARIES Consolidated Balance Sheets September 30, 2023 and December 31, 2022 (In thousands) | ||||||||||||||||
| September 30, 2023 | December 31, | |||||||||||||||
| (Unaudited) | 2022 | |||||||||||||||
| Current assets | ||||||||||||||||
| Cash and cash equivalents | $ | 12,632 | $ | 4,444 | ||||||||||||
| Accounts receivable, net of allowance for doubtful accounts and discounts & allowances of $1,430 and $1,820 at September 30, 2023 and December 31, 2022 respectively | 13,095 | 11,414 | ||||||||||||||
| Inventories, net | 9,321 | 9,631 | ||||||||||||||
| Prepaid expenses and other current assets | 1,621 | 1,445 | ||||||||||||||
| Refundable income taxes | 260 | 44 | ||||||||||||||
| Total current assets | 36,929 | 26,978 | ||||||||||||||
| Property, plant and equipment, net | 22,285 | 20,905 | ||||||||||||||
| Operating lease right-of-use asset | 203 | 174 | ||||||||||||||
| Goodwill | 11,704 | 11,704 | ||||||||||||||
| Intangible assets, net | 7,033 | 7,438 | ||||||||||||||
| Other assets | 1,900 | 1,800 | ||||||||||||||
| Total assets | $ | 80,054 | $ | 68,999 | ||||||||||||
| Current liabilities | ||||||||||||||||
| Current portion of note payable | $ | 1,250 | $ | 1,250 | ||||||||||||
| Accounts payable | 9,102 | 7,979 | ||||||||||||||
| Accrued expenses | 5,555 | 3,813 | ||||||||||||||
| Accrued income taxes | 500 | – | ||||||||||||||
| Total current liabilities | 16,407 | 13,042 | ||||||||||||||
| Line of credit | 2,777 | 2,777 | ||||||||||||||
| Note payable | 1,731 | 2,477 | ||||||||||||||
| Operating lease liabilities | 130 | 104 | ||||||||||||||
| Deferred income taxes, net | 3,029 | 3,029 | ||||||||||||||
| Total liabilities | 24,074 | 21,429 | ||||||||||||||
| Commitments and contingencies (Note 9) | – | – | ||||||||||||||
| Stockholders’ equity | ||||||||||||||||
| Preferred stock, no par value; 2,500 shares authorized; no shares issued or outstanding at September 30, 2023 and December 31, 2022 | – | – | ||||||||||||||
| Common stock, no par value; 40,000 shares authorized; 17,274 shares issued; 14,691 and 14,645 outstanding at September 30, 2023 and December 31, 2022, respectively | 6,509 | 6,509 | ||||||||||||||
| Paid-in capital | 4,338 | 3,624 | ||||||||||||||
| Treasury stock, at cost | (16,695) | (16,993) | ||||||||||||||
| Retained earnings | 61,828 | 54,430 | ||||||||||||||
| Total stockholders’ equity | 55,980 | 47,570 | ||||||||||||||
| Total liabilities and stockholders’ equity | $ | 80,054 | $ | 68,999 | ||||||||||||
| LIFEWAY FOODS, INC. AND SUBSIDIARIES Consolidated Statements of Operations For the three and nine months ended September 30, 2023 and 2022 (Unaudited) (In thousands, except per share data) | ||||||||||||||||
| Three Months Ended September 30, | Nine months Ended September 30, | |||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||
| Net sales | $ | 40,896 | $ | 38,140 | $ | 118,030 | $ | 105,730 | ||||||||
| Cost of goods sold | 29,099 | 29,962 | 85,428 | 85,032 | ||||||||||||
| Depreciation expense | 654 | 590 | 1,953 | 1,833 | ||||||||||||
| Total cost of goods sold | 29,753 | 30,552 | 87,381 | 86,865 | ||||||||||||
| Gross profit | 11,143 | 7,588 | 30,649 | 18,865 | ||||||||||||
| Selling expenses | 2,884 | 2,843 | 8,974 | 8,527 | ||||||||||||
| General and administrative | 3,085 | 3,415 | 10,028 | 9,546 | ||||||||||||
| Amortization expense | 135 | 135 | 405 | 405 | ||||||||||||
| Total operating expenses | 6,104 | 6,393 | 19,407 | 18,478 | ||||||||||||
| Income from operations | 5,039 | 1,195 | 11,242 | 387 | ||||||||||||
| Other income (expense): | ||||||||||||||||
| Interest expense | (109) | (77) | (322) | (171) | ||||||||||||
| Gain on sale of property and equipment | – | – | 33 | – | ||||||||||||
| Other (expense) income, net | (1) | (5) | (1) | (10) | ||||||||||||
| Total other income (expense) | (110) | (82) | (290) | (181) | ||||||||||||
| Income before provision for income taxes | 4,929 | 1,113 | 10,952 | 206 | ||||||||||||
| Provision (benefit) for income taxes | 1,517 | 130 | 3,554 | (2) | ||||||||||||
| Net income | $ | 3,412 | $ | 983 | $ | 7,398 | $ | 208 | ||||||||
| Earnings per common share: | ||||||||||||||||
| Basic | $ | 0.23 | $ | 0.06 | $ | 0.50 | $ | 0.01 | ||||||||
| Diluted | $ | 0.23 | $ | 0.06 | $ | 0.49 | $ | 0.01 | ||||||||
| Weighted average common shares: | ||||||||||||||||
| Basic | 14,677 | 15,490 | 14,659 | 15,462 | ||||||||||||
| Diluted | 15,101 | 15,848 | 15,063 | 15,759 | ||||||||||||
| LIFEWAY FOODS, INC. AND SUBSIDIARIES Consolidated Statements of Cash Flows (Unaudited) (In thousands) | ||||||||
| Nine months ended September 30, | ||||||||
| 2023 | 2022 | |||||||
| Cash flows from operating activities: | ||||||||
| Net income | $ | 7,398 | $ | 208 | ||||
| Adjustments to reconcile net income to operating cash flow: | ||||||||
| Depreciation and amortization | 2,358 | 2,238 | ||||||
| Stock-based compensation | 1,078 | 755 | ||||||
| Non-cash interest expense | 5 | 5 | ||||||
| Bad debt expense | 2 | – | ||||||
| Deferred revenue | – | (23) | ||||||
| Gain on sale of equipment | (33) | – | ||||||
| (Increase) decrease in operating assets: | ||||||||
| Accounts receivable | (1,683) | (1,576) | ||||||
| Inventories | 310 | (907) | ||||||
| Refundable income taxes | (216) | (309) | ||||||
| Prepaid expenses and other current assets | (176) | (115) | ||||||
| Increase (decrease) in operating liabilities: | ||||||||
| Accounts payable | 928 | 3,085 | ||||||
| Accrued expenses | 1,673 | 1,003 | ||||||
| Accrued income taxes | 500 | (725) | ||||||
| Net cash provided by operating activities | 12,144 | 3,639 | ||||||
| Cash flows from investing activities: | ||||||||
| Purchases of property and equipment | (3,146) | (2,609) | ||||||
| Proceeds from sales of equipment | 40 | – | ||||||
| Acquisition, net of cash acquired | – | (580) | ||||||
| Purchase of investments | (100) | – | ||||||
| Net cash used in investing activities | (3,206) | (3,189) | ||||||
| Cash flows from financing activities: | ||||||||
| Repayment of note payable | (750) | (750) | ||||||
| Net cash used in financing activities | (750) | (750) | ||||||
| Net increase (decrease) in cash and cash equivalents | 8,188 | (300) | ||||||
| Cash and cash equivalents at the beginning of the period | 4,444 | 9,233 | ||||||
| Cash and cash equivalents at the end of the period | $ | 12,632 | $ | 8,933 | ||||
| Supplemental cash flow information: | ||||||||
| Cash paid for income taxes, net | $ | 3,270 | $ | 640 | ||||
| Cash paid for interest | $ | 343 | $ | 158 | ||||
| Non-cash investing activities | ||||||||
| Accrued purchase of property and equipment | $ | 194 | $ | 250 | ||||
| Increase in right-of-use assets and operating lease obligations | $ | 86 | $ | 19 | ||||
SOURCE Lifeway Foods, Inc.