Image Credit: The Focal Project (Flickr)
Sixteen Years After the Human Genome Project Completion, Regenerative Medicine May Hit its Stride
Bluebird Bio’s performance this past week shows what the power of FDA approval can do when a biotech company gets the green light. Not only did investors enjoy a 51.7% increase in the gene therapy company’s stock price, but patients can now overcome a severe genetic disease. Gene therapies and regenerative medicine are now beginning to blossom, 16 years after the last human chromosome was mapped, completing the human genome project. Quite a few specialized biotech companies have been started within the past 16 years, and many have a product pipeline in various stages of FDA approval. Below is information on Bluebird and two other public companies involved in genetic medicine that you may want to pay attention to.
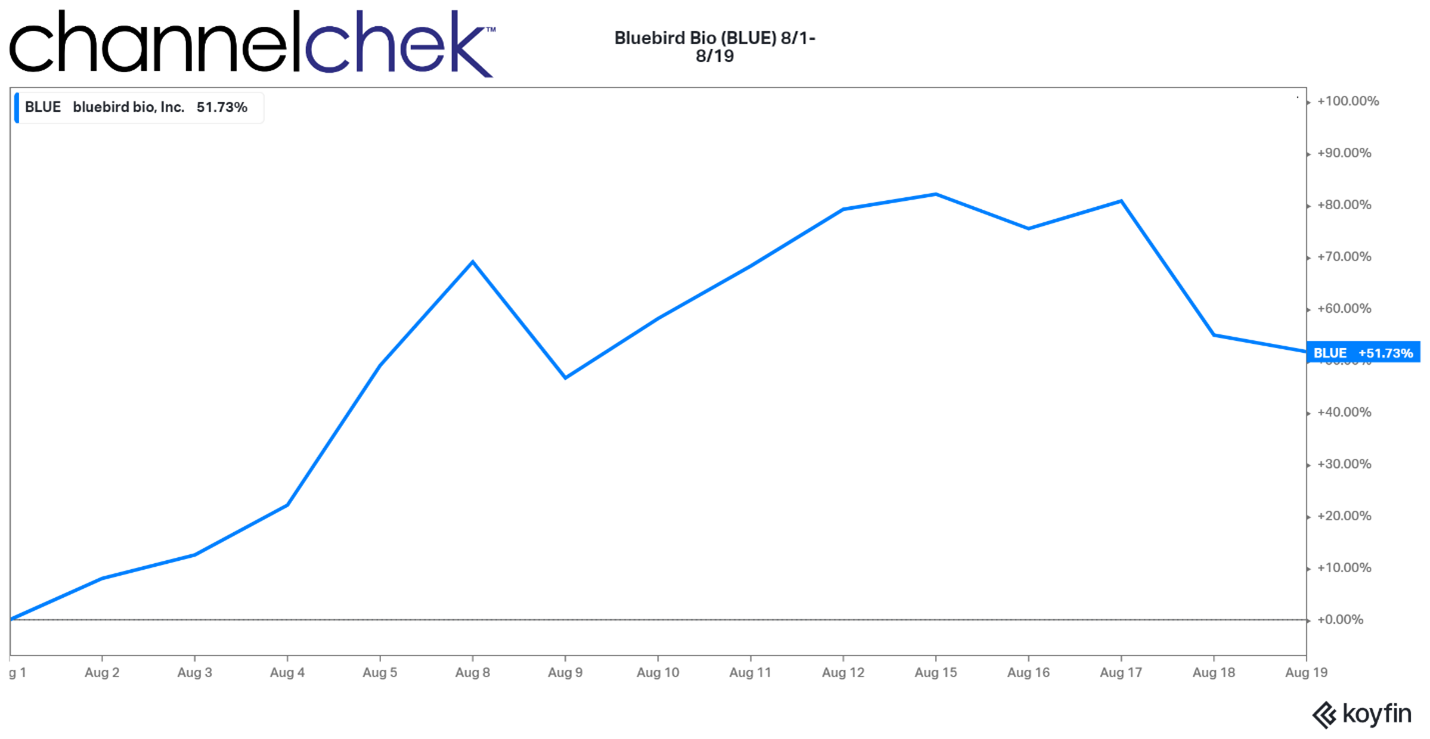
Source: Koyfin
Bluebird Bio (BLUE)
Bluebird Bio’s blood disorder gene therapy was approved a few days earlier than anticipated. The approval of Zynteglo is exciting for those whose lives will be changed by the gene therapy, investors that have had confidence in the surrounding science, and of course, everyone working at Bluebird bio. Congratulations.
A downside to the FDA approval is that the therapy for betibeglogene autotemcel (beti-cel), will initially cost $2.8 million for those receiving it. More positively, the pediatric and adult patients that will benefit have been requiring ongoing red blood cell transfusions. Zynteglo is a one-time gene therapy, so those afflicted can receive transfusion independence.
Lineage Cell Therapeutics (LCTX)
Lineage Cell Therapeutics is a clinical-stage biotech company developing new cellular therapies for degenerative retinal diseases, neurological conditions associated with demyelination, and helping the body fight cancer. At the company’s core are two proprietary technology platforms: cell replacement and cell and drug delivery. Its cell replacement platform creates new cells and tissues with its pluripotent and progenitor cell technologies. The company’s cell and drug delivery programs are based upon its proprietary HyStem cell and drug delivery matrix technology.
There is no better way for any investor to develop an understanding of a biotech company in the regenerative medicine or gene therapy space than to have its products and pipeline explained to them by the CEO. Channelchek and Noble Capital Markets have arranged for an online interactive roadshow (bring your questions) for interested investors to attend. This will be held on Tuesday (August 23). More information is available here.
Miromatrix (MIRO)
Miromatrix is a company that may one day eliminate waitlists for organ transplants. The company claims to lead the development of bioengineered organs for transplantation with over 118 issued patents worldwide. The technology is able to be applied across many of needs. Specifically, they are currently focused on creating transplantable kidneys (MiroKidney) and livers (MiroLiver) with expectations to also bioengineer other critical organs like lungs, pancreas, and heart.
The decellularization technology is supported through pre-clinical and animal studies that the FDA cleared in previously commercialized matrix products. The company’s bioengineered organs have the potential to eliminate ongoing therapies and prolong life. They also are expected to reduce costs across many areas of the healthcare system.
Take Away
The FDA approval of Zynteglo for Bluebird Bio and the stock performance was a reminder this week of the potential for companies in the regenerative medicine space to make a difference. It has been 16 years since the human genome project was completed. Many of these companies were started shortly after; perhaps their research and development are about to pay off.
Explore more companies like BLUE, LCTX, and MIRO by registering for Channelchek and exploring under the “COMPANY Data” tab.
Managing Editor, Channelchek

|
Virtual Roadshow – August 23 Lineage Cell Therapeutics – Brian M. Culley, CEOJoin Lineage Cell Therapeutics CEO Brian M. Culley for this exclusive corporate presentation, followed by a Q & A session moderated by Robert LeBoyer, Noble’s senior research analyst, featuring questions taken from the audience. Registration is free and open to all investors, at any level Register Now
|
Suggested Content
 Stem Cell-Derived Retinal Pigment Epithelium Cells – Vision for the Future
|
 Preventing the Immune System from Rejecting Gene Therapy
|
 Pros and Cons of FDA Funded in Part by Companies
|
 Cells that Can be Produced from Stem Cells
|
Source
https://en.wikipedia.org/wiki/Human_Genome_Project#History
Stay up to date. Follow us:

|
