
Research News and Market Data on ESEA
February 11, 2026 09:00 ET | Source: Euroseas
ATHENS, Greece, Feb. 11, 2026 (GLOBE NEWSWIRE) — Euroseas Ltd. (NASDAQ: ESEA, the “Company” or “Euroseas”), an owner and operator of container carrier vessels and provider of seaborne transportation for containerized cargoes, announced today a new time charter contract for its 2007-built 1,740 teu feeder containership, EM Spetses, for a minimum period of 22 to a maximum period of 24 months, at the option of the charterer, at a gross daily rate of $21,500. The new charter period will commence on April 12, 2026, in direct continuation of its present charter, and represents a daily increase of over $3,000 over the vessel’s current rate.
Aristides Pittas, Chairman and CEO of Euroseas, commented: “We are very pleased to that we have extended the time charter contract for our 2007-built EM Spetses with a top-class charterer, in direct continuation of its present charter, for 22-24 months at a profitable rate of $21,500. This fixture highlights that despite the upcoming Lunar New Year holidays, activity across the feeder segment remains firm, as operators move to secure their requirements amid a tight container chartering market with very limited tonnage availability. The charter is expected to generate about $8.9 million of EBITDA over the minimum contracted period and increases our charter coverage for 2026, 2027, and 2028 to about 87%, 71% and 41% respectively.”
Fleet Profile:
The Euroseas Ltd. fleet profile is currently as follows:
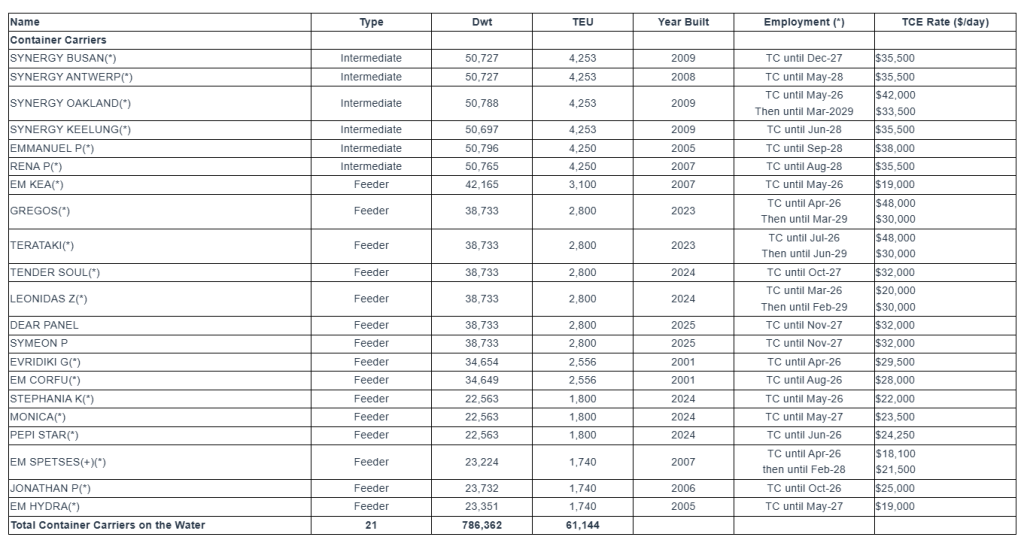
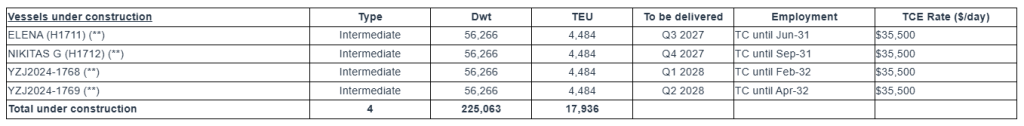
Notes:
(*)TC denotes time charter. Charter duration indicates the earliest redelivery date; all dates listed are the earliest redelivery dates under each TC unless the contract rate is lower than the current market rate in which cases the latest redelivery date is assumed; vessels with the latest redelivery date shown are marked by (+).
(**) The charterer has the option until Nov-2026 to extend the charters by one year with the rate for the five-year period becoming $32,500/day.
About Euroseas Ltd.
Euroseas Ltd. was formed on May 5, 2005 under the laws of the Republic of the Marshall Islands to consolidate the ship owning interests of the Pittas family of Athens, Greece, which has been in the shipping business over the past 150 years. Euroseas trades on the NASDAQ Capital Market under the ticker ESEA.
Euroseas operates in the container shipping market. Euroseas’ operations are managed by Eurobulk Ltd., an ISO 9001:2008 and ISO 14001:2004 certified affiliated ship management company, which is responsible for the day-to-day commercial and technical management and operations of the vessels. Euroseas employs its vessels on spot and period charters and through pool arrangements.
The Company has a fleet of 21 vessels, including 15 Feeder containerships and 6 Intermediate containerships with a cargo capacity of 61,144 teu. After the delivery of four intermediate containership newbuildings in 2027 and 2028, respectively, Euroseas’ fleet will consist of 25 vessels with a total carrying capacity of 79,080 teu.
Forward Looking Statement
This press release contains forward-looking statements (as defined in Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended) concerning future events and the Company’s growth strategy and measures to implement such strategy; including expected vessel acquisitions and entering into further time charters. Words such as “expects,” “intends,” “plans,” “believes,” “anticipates,” “hopes,” “estimates,” and variations of such words and similar expressions are intended to identify forward-looking statements. Although the Company believes that the expectations reflected in such forward-looking statements are reasonable, no assurance can be given that such expectations will prove to have been correct. These statements involve known and unknown risks and are based upon a number of assumptions and estimates that are inherently subject to significant uncertainties and contingencies, many of which are beyond the control of the Company. Actual results may differ materially from those expressed or implied by such forward-looking statements. Factors that could cause actual results to differ materially include, but are not limited to changes in the demand for containerships, competitive factors in the market in which the Company operates; risks associated with operations outside the United States; and other factors listed from time to time in the Company’s filings with the Securities and Exchange Commission. The Company expressly disclaims any obligations or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in the Company’s expectations with respect thereto or any change in events, conditions or circumstances on which any statement is based.
Visit our website www.euroseas.gr
| Company Contact | Investor Relations / Financial Media |
| Tasos Aslidis Chief Financial Officer Euroseas Ltd. 11 Canterbury Lane, Watchung, NJ 07069 Tel. (908) 301-9091 E-mail: aha@euroseas.gr | Nicolas Bornozis Markella Kara Capital Link, Inc. 230 Park Avenue, Suite 1540 New York, NY 10169 Tel. (212) 661-7566 E-mail: euroseas@capitallink.com |


