Research News and Market Data on OCGN
November 9, 2023
Conference Call and Webcast Today at 8:30 a.m. ET
- OCU400 demonstrated favorable safety and tolerability profile in retinitis pigmentosa (RP) and Leber congenial amaurosis (LCA) subjects
- Completed dosing of three LCA patients including a pediatric patient
- OCU400 Phase 1/2 study results suggest stabilization or improvement of Best-Corrected Visual Acuity (BCVA) or Multi-Luminance Mobility Testing (MLMT) or Low-Luminance Visual Acuity (LLVA) in treated eyes of 83% (10/12) subjects
- Stabilization or improvement in MLMT scores from baseline in 86% (6/7) of RHO subjects demonstrated gene-agnostic property of OCU400 modifier gene therapy and its potential benefits in broader RP and LCA subjects
- Ocugen’s inhaled mucosal vaccine candidate for COVID-19—OCU500—selected by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases’ (NIAID) Project NextGen for inclusion in clinical trials
MALVERN, Pa., Nov. 09, 2023 (GLOBE NEWSWIRE) — Ocugen, Inc. (Ocugen or the Company) (NASDAQ: OCGN), a biotechnology company focused on discovering, developing, and commercializing novel gene and cell therapies, biologics, and vaccines, today reported third quarter 2023 financial results along with a general business update.
“Ocugen has made significant pipeline progress in the third quarter,” said Dr. Shankar Musunuri, Chairman, Chief Executive Officer, and Co-Founder of Ocugen. “In particular, based on the OCU400 data presented in September, I am as enthusiastic as ever regarding the potential for our modifier gene therapy approach to make an important difference in the lives of people living with blindness diseases. OCU400—our lead candidate—has the potential to address multiple genetic mutations with a single product compared to traditional gene therapies that target one gene at a time.”
This clinical efficacy update provided in September 2023 included data for 12 subjects who have completed a minimum of 6 months follow up. 83%, 83%, and 75% of subjects demonstrated stabilization or improvements in OCU400 treated eyes on BCVA, LLVA, and MLMT scores, respectively from baseline. 86% of subjects with the RHO gene mutation experienced either stabilization or increase in MLMT scores from baseline, including a subset of 29% that demonstrated a three Lux luminance level improvement. Preservation of remaining vision, slowing disease progression, or improving the vision can significantly impact patients’ quality of life.
“The improvements in BCVA, LLVA and MLMT in RHO patients—a disease affecting more than 10,000 people in the U.S. alone—supports the gene-agnostic mechanism of action for OCU400,” said Dr. Musunuri.
In October 2023, OCU500, was selected by the NIAID Project NextGen for inclusion in clinical trials. OCU500 will be tested via two different mucosal routes, inhalation into the lungs and as a nasal spray. Currently used injected vaccines, including mRNA vaccines, are not effective in preventing infection and spread although effective against severe infection. Generating mucosal immunity in nasal and respiratory airways could help block the infection at its origin, thus limiting spread.
“NIAID support for our mucosal vaccine platform is the result of many months of hard work and dedicated effort by our Ocugen team and is a first step in potentially expanding the platform to flu and other respiratory viral diseases and infections,” said Dr. Musunuri. “Additionally, this funding makes it possible to focus the majority of Ocugen’s R&D and clinical resources on our first-in-class gene and cell therapies.”
As Ocugen prepares to start Phase 3 for OCU400, begin dosing patients for OCU410 and OCU410ST, and initiate the Phase 1 trial for OCU500 in collaboration with NIAID, the company is making meaningful progress toward its long-term strategy and delivering on each of its scientific platforms in the near-term.
Ophthalmic Gene Therapies—First-in-class
- OCU400 – Completed dosing adult RP patients in the dose-escalation and dose-expansion portions of the trial; completed dosing three LCA patients including a pediatric patient. Phase 3 clinical trial for the treatment of RP to be initiated in early 2024 following FDA concurrence on study design. Subsequently, the Company is expecting to expand the OCU400 Phase 3 clinical trial for LCA patients in the second half of 2024 based on Phase 1/2 study results in LCA patients and alignment with the FDA.
- OCU410 and OCU410ST – IND applications to initiate Phase 1/2 trials for both OCU410 and OCU410ST were cleared by the FDA and the Company intends to dose patients in Phase 1/2 trials by the end of 2023.
Ophthalmic Biologic Product
- OCU200 – Continuing to work on the Company’s response to the FDA regarding the IND application and expect to initiate the Phase 1 clinical trial in the first half of 2024, contingent on the lift of the FDA hold and adequate availability of funding.
Regenerative Cell Therapies—First-in-class
- NeoCart® – Ocugen’s autologous regenerative cell therapy (using patients’ own cartilage cells) remains on track to begin its Phase 3 clinical trial in the second half of 2024. A cGMP facility for manufacturing NeoCart is expected to be completed at the end of 2023 and qualification is expected in the first half of 2024.
Vaccines Portfolio—First-in-class
- Mucosal Vaccine Platform – The Company is collaborating with NIAID to initiate clinical trials of OCU500 in early 2024.
Third Quarter 2023 Financial Results
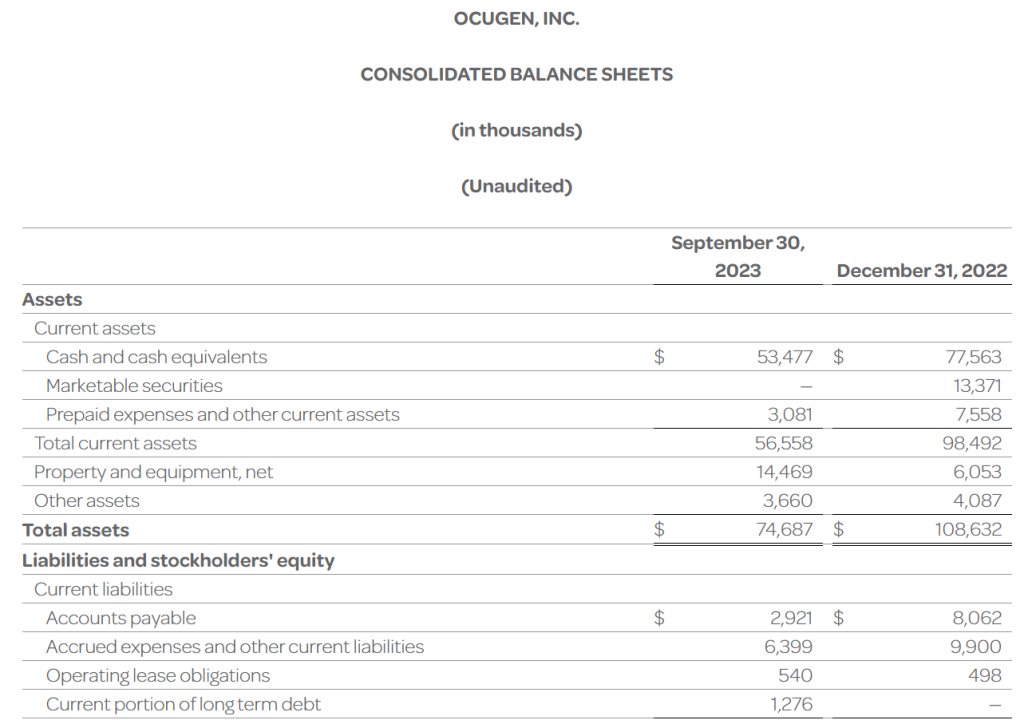
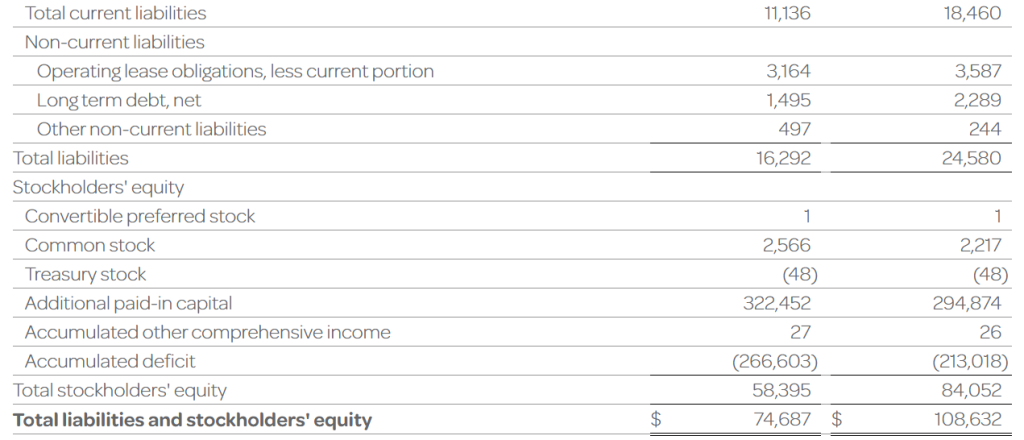
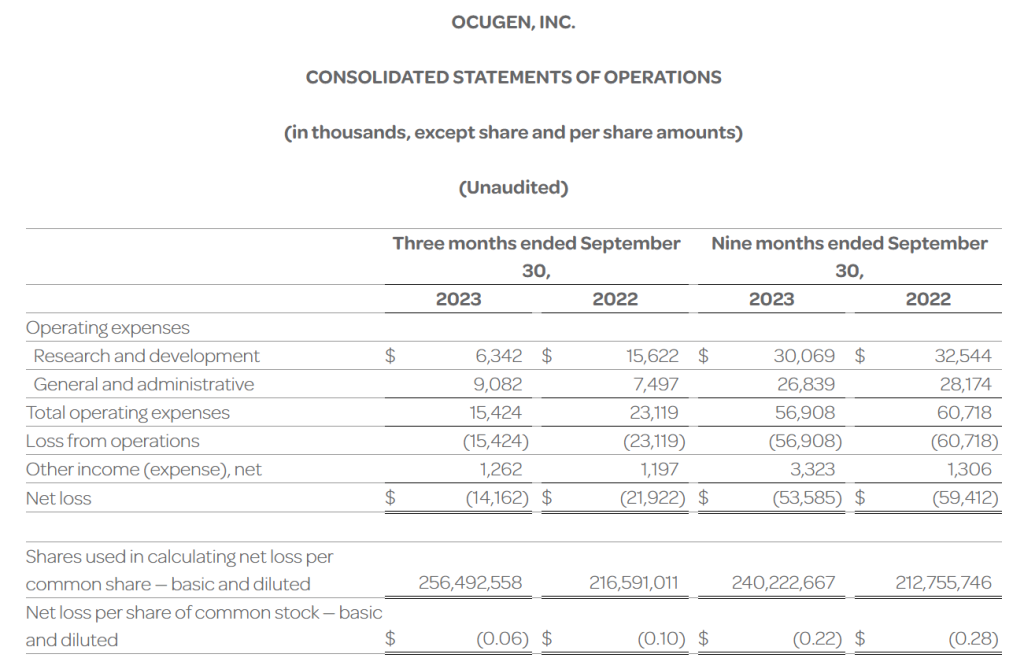
- The Company’s cash, cash equivalents, and investments totaled $53.5 million as of September 30, 2023, compared to $90.9 million as of December 31, 2022. The Company had 256.5 million shares of common stock outstanding as of September 30, 2023.
- Total operating expenses for the three months ended September 30, 2023 were $15.4 million and included research and development expenses of $6.3 million and general and administrative expenses of $9.1 million. This compares to total operating expenses for the three months ended September 30, 2022 of $23.1 million that included research and development expenses of $15.6 million and general and administrative expenses of $7.5 million.
- Ocugen reported a $0.06 net loss per common share for the three months ended September 30, 2023 compared to a $0.10 net loss per common share for the three months ended September 30, 2022.
Conference Call and Webcast Details
Ocugen has scheduled a conference call and webcast for 8:30 a.m. ET today to discuss the financial results and recent business highlights. Ocugen’s senior management team will host the call, which will be open to all listeners. There will also be a question-and-answer session following the prepared remarks.
Attendees are invited to participate on the call or webcast using the following details:
Dial-in Numbers: (800) 715-9871 for U.S. callers and (646) 307-1963 for international callers
Conference ID: 1787631
Webcast: Available on the events section of the Ocugen investor site
A replay of the call and archived webcast will be available for approximately 45 days following the event on the Ocugen investor site.
About Ocugen, Inc.
Ocugen, Inc. is a biotechnology company focused on discovering, developing, and commercializing novel gene and cell therapies, biologics, and vaccines that improve health and offer hope for patients across the globe. We are making an impact on patient’s lives through courageous innovation—forging new scientific paths that harness our unique intellectual and human capital. Our breakthrough modifier gene therapy platform has the potential to treat multiple retinal diseases with a single product, and we are advancing research in infectious diseases to support public health and orthopedic diseases to address unmet medical needs. Discover more at www.ocugen.com and follow us on X and LinkedIn.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995, which are subject to risks and uncertainties. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements include, but are not limited to, statements regarding the Company’s clinical development activities and related anticipated timelines; strategy, business plans and objectives for its clinical stage programs; plans and timelines for the preclinical and clinical development of its product candidates, including the therapeutic potential, clinical benefits and safety thereof; expectations regarding timing, success and data announcements of current ongoing preclinical and clinical trials; the ability to initiate new clinical programs; and Ocugen’s financial condition. Such statements are subject to numerous important factors, risks, and uncertainties that may cause actual events or results to differ materially from our current expectations. These and other risks and uncertainties are more fully described in our periodic filings with the Securities and Exchange Commission (SEC), including the risk factors described in the section entitled “Risk Factors” in the quarterly and annual reports that we file with the SEC, as well as discussions of potential risks, uncertainties, and other important factors in Ocugen’s subsequent filings with the SEC. Any forward-looking statements that we make in this press release speak only as of the date of this press release and should not be relied upon as representing its views as of any subsequent date. Except as required by law, we assume no obligation to update forward-looking statements contained in this press release whether as a result of new information, future events, or otherwise, after the date of this press release.
Contact:
Tiffany Hamilton
Head of Communications
IR@ocugen.com