
Research, News, and Market Data on AXLA
November 1, 2022 at 7:00 AM EDT
- Promising Results from Phase 2a Placebo Controlled Clinical Trial for Long COVID
- Positive Interim Data from Phase 2b EMMPACT Study of AXA1125 in Nonalcoholic Steatohepatitis (NASH)
- $34.2 Million Registered Direct Offering of Common Stock Priced At The Market
- Appointment of Two New Board Members
CAMBRIDGE, Mass.–(BUSINESS WIRE)–Nov. 1, 2022– Axcella Therapeutics (Nasdaq: AXLA), a clinical-stage biotechnology company pioneering a new approach to treat complex diseases using multi-targeted endogenous metabolic modulator (EMM) compositions, today announced financial results for the third quarter ended September 30, 2022 and provided a business update.
“This has been an extremely exciting period for Axcella,” said Bill Hinshaw President and Chief Executive Officer of Axcella. “Through the third quarter, we made important progress in our clinical development of AXA1125 and strengthened our balance sheet with a new financing. Specifically, we reported positive data from both our Phase 2 study of Long COVID and our Phase 2b EMMPACT study in non-alcoholic steatohepatitis (NASH). In addition, we completed a financing worth $34.2 million that will allow us to advance the Long COVID program and our EMMPACT Phase 2b clinical trial in NASH.”
Mr. Hinshaw continued, “As the company advances toward late-stage clinical trials, we are pleased to have the additions of Mr. Rosiello and Mr. Straight Nissen to our Board, whose extensive leadership experience will help guide Axcella as we focus on taking steps toward delivering these treatments to patients.”
Clinical Results in the Quarter:
The quarter saw progress in the clinical development of AXA1125, an orally delivered potent and safe compound addressing two very large markets: Long-COVID and NASH. This included the release of top-line results in Long COVID and an interim analysis of the Phase 2b EMMPACT trial in NASH.
Long COVID is a persistent and growing challenge of the pandemic, affecting an estimated one hundred million patients worldwide, with fatigue as the most common symptom. In a release of top-line results, subjects who received AXA1125 had improvements in measures of mental and physical fatigue that were both highly statistically significant and clinically relevant compared to those who received placebo. Mean changes in total, physical and mental scores in the CFQ-11 versus placebo were -4.30 (p=0.0039), -2.94 (p=0.0097) and -1.32 (p=0.0097), respectively.
NASH affects an estimated 40 million people in the U.S., including approximately 10% of U.S. children. The complex pathogenesis of NASH includes dysregulation of metabolism, inflammation and fibrosis. In this interim analysis, at 24-weeks there were statistically significant improvements in the liver stiffness measurement (LSM) using vibration-controlled transient elastography compared to placebo in the high dose arm for all subjects. Absolute changes in LSM were 0.13, -2.01, and -4.07 kilopascals (kPa) in the placebo, low dose and high dose arms, respectively (p= 0.0992 and 0.0096 for the low and high dose, respectively, compared to placebo). These results were supported by statistically significant improvements in other non-invasive measures of liver fibrosis: ELF and FIB-4. Statistically significant improvements in alanine aminotransferase (ALT) were seen at both weeks 12 and 24 in all subjects (placebo-adjusted difference of -28.61% (p=0.0183) and -36.3% (p=0.0017) for the low and high doses, respectively). All subjects experienced significantly greater changes from baseline in MRI-PDFF at 12-weeks compared to the change from baseline in the placebo group (placebo adjusted difference of -18.98% (p=0.0082) and -21.24% (p=0.0014) for the low and high doses, respectively).
Financing and Board:
In September 2022, the Company received $6.0 million from the issuance of convertible notes to funds associated with Flagship Pioneering. In October 2022, the Company secured an additional $28.2 million in gross proceeds through a registered direct offering of common stock, which also resulted in the conversion of the convertible notes into common stock. In total, the Company sold 20,847,888 shares of common stock at a purchase price of $1.64 per share. Since this offering was made without an underwriter or a placement agent, Axcella did not pay any underwriting or placement agent commissions. Simultaneous with the financing, the company appointed Robert Rosiello and Torben Straight Nissen to its Board of Directors, and Mr. Rosiello also became Chairman of the Board. After five years of service, David Epstein stepped off the Board, and will continue to support the Company as a consultant.
Financial Results:
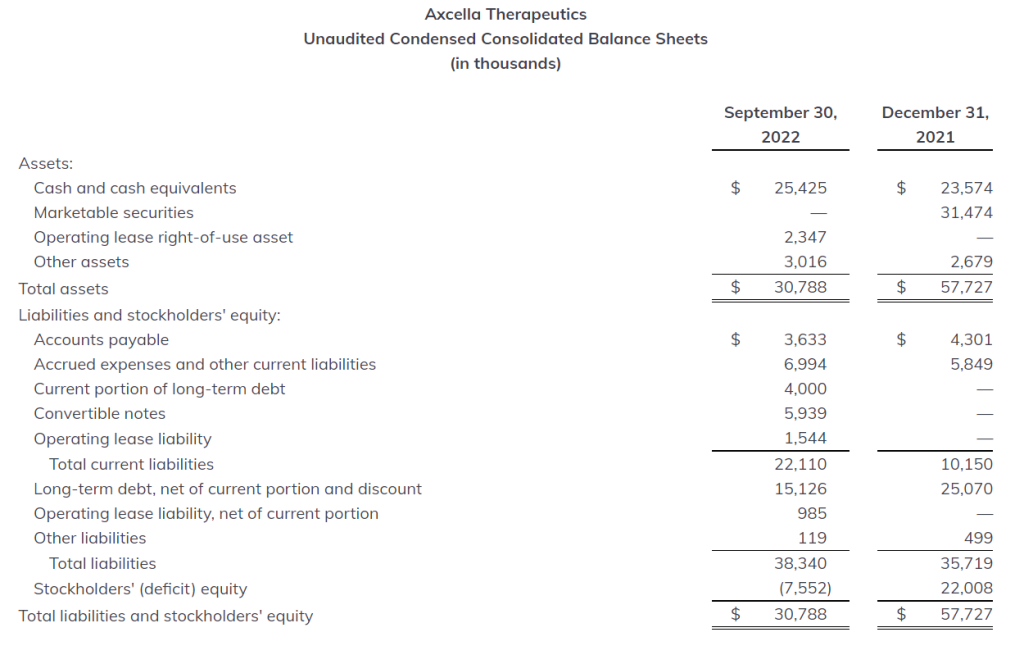
Cash Position: As of September 30, 2022, cash, cash equivalents, and marketable securities totaled $25.4 million, compared to $55.0 million as of December 31, 2021. In September 2022, the Company received $6.0 million from the issuance of convertible notes to funds associated with Flagship Pioneering and, in October 2022, the Company secured an additional $28.2 million in gross proceeds through a registered direct offering of common stock. As part of the registered direct offering, the $6.0 million convertible notes automatically converted into the Company’s common stock. Axcella expects that its current cash balance will be sufficient to meet its operating needs into the second quarter of 2023, provided that, if the Company is unable to satisfy the cash covenants contained in its loan and security agreement with SLR Investment Corp., and SLR Investment Corp. seeks immediate repayment of the loan in full, the Company believes that its cash and cash equivalents will be sufficient to fund its operations into the late first quarter of 2023.
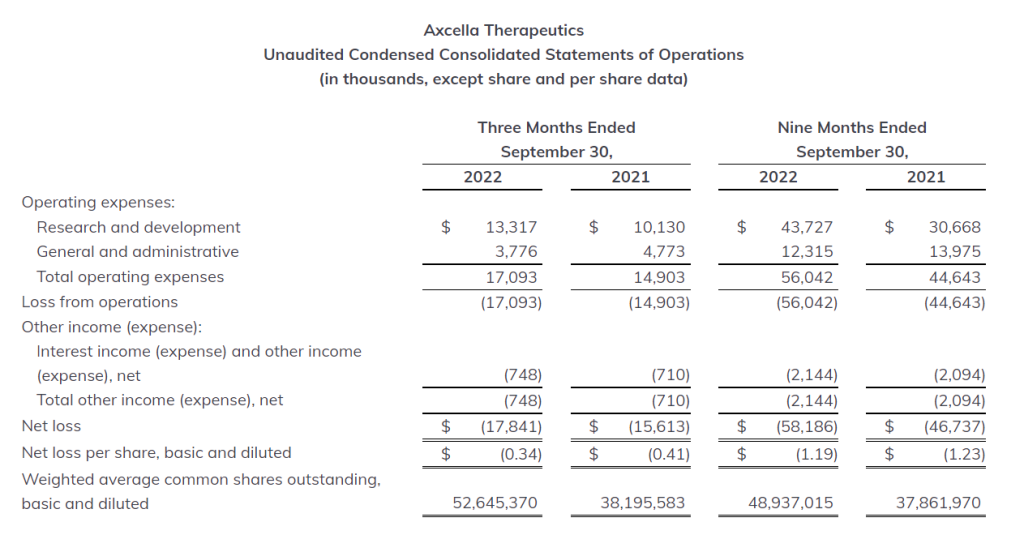
R&D Expenses: Research and development expenses for the quarter and nine months ended September 30, 2022 were $13.3 million and $43.7 million, respectively. Research and development expenses for the same periods ended September 30, 2021 were $10.1 million and $30.7 million, respectively. These increases are the result of the Company’s EMMPACT and Long COVID Phase 2 clinical trials, as well as closure costs for its EMMPOWER Phase 2 clinical trial.
G&A Expenses: General and administrative expenses for the quarter and nine months ended September 30, 2022 were $3.8 million and $12.3 million, respectively. General and administrative expenses for the same periods ended September 30, 2021 were $4.8 million and $14.0 million. These decreases are primarily the result of lower non-cash stock-based compensation expenses.
Net Loss: Net loss for the quarter and nine months ended September 30, 2022 was $17.8 million, or $0.34 per basic and diluted share, and $58.2 million, or $1.19 per basic and diluted share, respectively. This compares with a net loss of $15.6 million, or $0.41 per basic and diluted share, and $46.7 million, or $1.23 per basic and diluted share, for the quarter and nine months ended September 30, 2021.
Internet Posting of Information
Axcella uses the “Investors and News” section of its website, www.axcellatx.com, as a means of disclosing material nonpublic information, to communicate with investors and the public, and for complying with its disclosure obligations under Regulation FD. Such disclosures include, but may not be limited to, investor presentations and FAQs, Securities and Exchange Commission filings, press releases, and public conference calls and webcasts. The information that we post on our website could be deemed to be material information. As a result, we encourage investors, the media and others interested to review the information that we post there on a regular basis. The contents of our website shall not be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended.
About Axcella Therapeutics (Nasdaq: AXLA)
Axcella is a clinical-stage biotechnology company pioneering a new approach to treat complex diseases using compositions of endogenous metabolic modulators (EMMs). The company’s product candidates are comprised of EMMs and derivatives that are engineered in distinct combinations and ratios to restore cellular homeostasis in multiple biological pathways, improve cellular energetics and restore homeostasis. Axcella’s pipeline includes lead therapeutic candidates in Phase 2 development for the treatment of Long COVID, and NASH. The company’s unique model allows for the evaluation of its EMM compositions through non-IND clinical studies or IND clinical trials. For more information, please visit www.axcellatx.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, statements regarding the timing of the company’s clinical trial data readouts, its expected cash runway and the expected benefits of Mr. Rosiello’s and Mr. Straight Nissen’s service on the Board of Directors of Axcella. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements in this press release are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this press release, including, without limitation, those related to the potential impact of COVID-19 on the company’s ability to conduct and complete its ongoing or planned clinical studies and clinical trials in a timely manner or at all due to patient or principal investigator recruitment or availability challenges, clinical trial site shutdowns or other interruptions and potential limitations on the quality, completeness and interpretability of data the company is able to collect in its clinical trials of AXA1125, other potential impacts of COVID-19 on the company’s business and financial results, including with respect to its ability to raise additional capital and operational disruptions or delays, changes in law, regulations, or interpretations and enforcement of regulatory guidance, whether data readouts support the company’s clinical trial plans and timing, clinical trial design and target indications for AXA1125, the clinical development and safety profile of AXA1125 and its therapeutic potential, whether and when, if at all, the company’s product candidates will receive approval from the FDA or other comparable regulatory authorities, potential competition from other biopharma companies in the company’s target indications, and other risks identified in the company’s SEC filings, including Axcella’s Annual Report on Form 10-K, Quarterly Report on Form 10-Q and subsequent filings with the SEC. The company cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. Axcella disclaims any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. Any forward-looking statements contained in this press release represent the company’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date. The company explicitly disclaims any obligation to update any forward-looking statements.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221101005428/en/
Company
Ashley Robinson
arr@lifesciadvisors.com
(617) 430-7577
Source: Axcella Therapeutics
