
Are Pluripotent Stem Cell Based Therapies Close to Market?
The history of deriving embryonic stem cells from mice goes back to its discovery in 1981. This was followed by a decade of research on mouse stem cell biology, which led scientists to find a method to derive stem cells from human embryos. In 1998 they developed a method to grow them in a laboratory setting. This was followed by another breakthrough in 2006; the identification of conditions allowing some specialized adult cells to be genetically reprogrammed to assume a stem cell-like state. These cells are called induced pluripotent stem cells (iPSCs). Shinya Yamanaka (Kyoto University, Japan and Gladstone Institutes, USA) and John Gurdon (Gurdon Institute, Cambridge, UK) received the Nobel Prize for Physiology or Medicine for the discovery of mature cells reprogrammed into a pluripotent state in 2012.
Many discoveries and milestones in the field ushered in a transition from fundamental research to pre-clinical research, and then to clinical trials. The history of stem cell research is highlighted in Exhibit 1. PSCs entered into the clinic first for the treatment of advanced macular degeneration in 2017 (Mandai et al. 2017).
Exhibit 1. The history of stem cell research
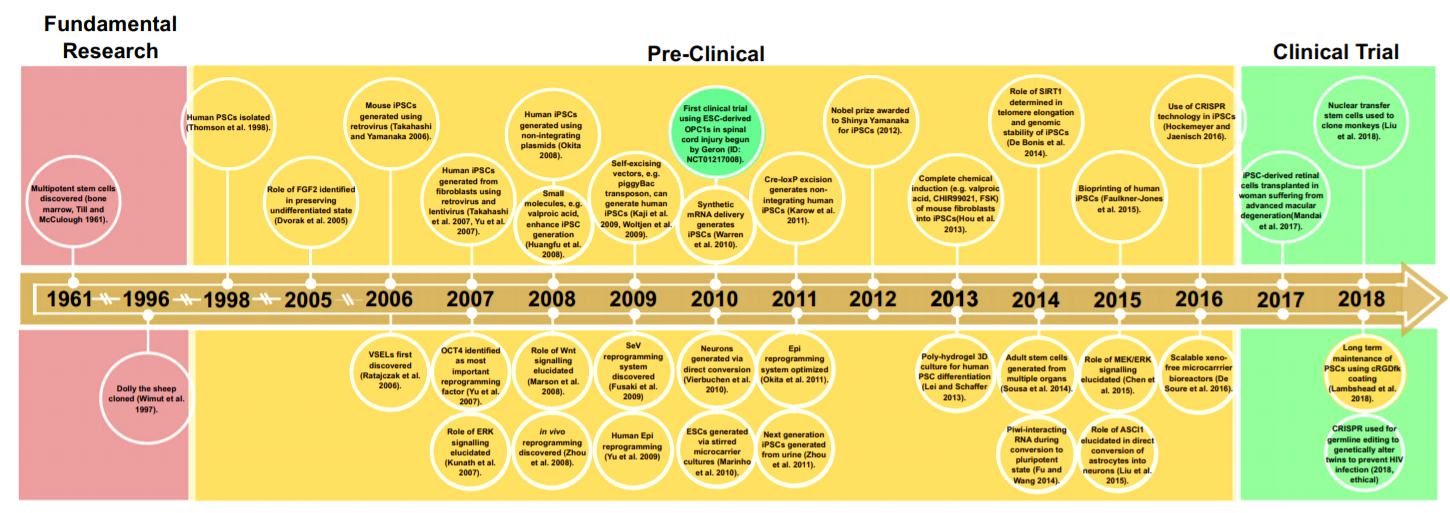
Source: Stem Cell Rev and Rep (2020) 16:3 –32
PSCs can function like embryonic stem (ES) cells and have the ability to differentiate or develop into a variety of specific cell types in the body, such as liver cells, muscle cells, blood cells (Exhibit 2). Stem-cell-based therapeutics is widely considered as a promising and exciting arena in medicine attributed to their capability of regenerating and repairing damaged tissues.
Exhibit 2. Stem cells can differentiate into any type of
cell in the body
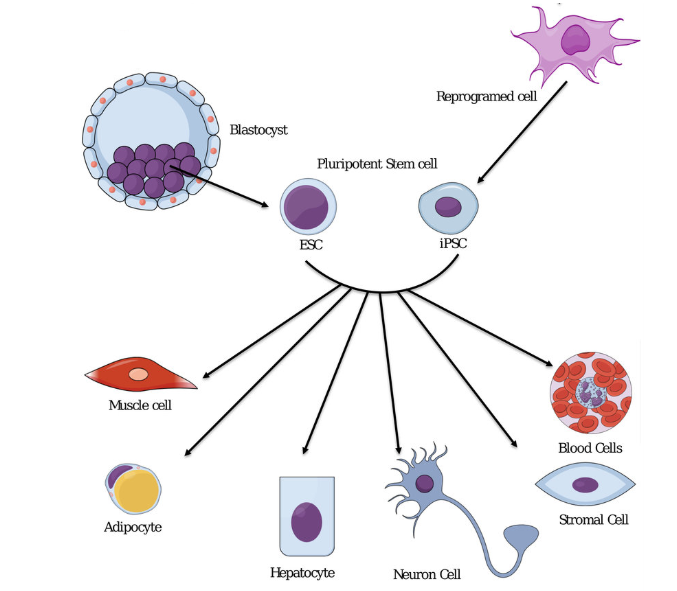
Source: SM. Afify et al. Cancers 2019, 11, 345
The transplantation into patients presents unique safety risks of human PSC (hPSC)-derived cell therapies. The major risks include i) hPSC differentiation yielding to a heterogeneous cell population, ii) residual undifferentiated hPSCs (10,000 or even fewer) forming a teratoma (a tumor made up of several different types of tissue, such as hair, muscle, teeth, or bone).
Exhibit 3. Safety risks of pluripotent cell-based
therapies
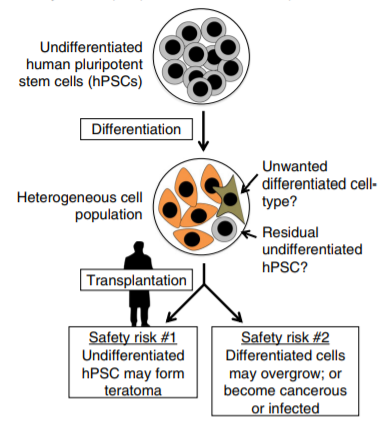
Source: RM. Martin ET AL. Nature Communications, (2020) 11:2713
Many hurdles and limitations in the production of clinical-grade embryonic stem cells (ESC) and iPSC derivatives have since been overcome. These cells can now be expanded in scalable suspension culture, a targeted, robust, and efficient differentiation of human ESCs and iPSCs can be achieved via inhibition and activation of molecular differentiation pathways.
Over the past 20 years, significant developmental milestones have driven basic, translational, and clinical advances in the fields of stem cells and regenerative medicine. Over the past decade, human pluripotent stem cell (hPSC)- derived cell therapies have been assessed in over 30 ongoing or completed clinical trials for various indications -including spinal cord injury, macular degeneration, and type 1 diabetes. Due to the immune privilege, the vast majority of the current clinical trials on transplantation of PSC-based cell products aim to treat macular degeneration. The eye is an “immune privileged” site. This phrase was defined by Peter Medawar and colleagues in the 1940s following the skin allografts placed within the anterior chamber of the eye surviving indefinitely, while their rapid rejection in other tissues such as the skin. The ocular microenvironment is highly anti-inflammatory. Multiple factors -such as proteins, neuropeptides, and biochemicals- modify the behavior, differentiation, and survival of immune cells within the ocular microenvironment promoting an anti-inflammatory, immune response, and induction of immune tolerance, which protects the eye from the irreversible collateral damage of inflammation that can lead to blindness.
Cellular therapies based on iPSCs are considered innovative but complex therapeutic concepts. The human pluripotent stem cell (hPSC)- derived cell therapies have rapidly expanded potential as therapeutics; however, they continue to carry safety risks. The scientific community has teamed up for the development of therapeutic applications and cellular products. The identification of the right patient population/indication and discovery of the optimum delivery route are crucial components for successful hPSC-derived cell therapy. Considering the scientific advancement in the genome editing approaches to the platform’s engineering, imaging, and other research and clinical tools, it may be a matter of time for hPSC-derived cell therapies to become a reality and lead to another breakthrough in medicine.
Suggested:
Enjoy the
Benefits of Premium Channelchek
Content at No
Cost

Each event in our popular Virtual Road Shows Series has maximum capacity of 100 investors online. To take part, listen to and perhaps get your questions answered, see which virtual investor meeting intrigues you here.