
|

|

|

|

New Era of Gene Therapy
(Note: companies that
could be impacted by the content of this article are listed at the base of the
story [desktop version]. This article uses third-party references to provide a
bullish, bearish, and balanced point of view; sources are listed after the
Balanced section.)
Gene therapy is a biological product that mediates its effect by transcription and/or translation of transferred genetic material by integrating into the host genome. It is administered as nucleic acids, viruses, or genetically engineered microorganisms. In gene therapy, a normal gene is inserted into the genome, often via a viral vector, to replace an abnormal gene responsible for causing a certain disease. The first clinical protocol introducing a foreign gene into humans was approved by the Recombinant DNA Advisory Committee (RAC) in December 1988. On September 14th, 1990, the U.S. Food and Drug Administration (FDA) approved for the first time treatment of a human patient with an experimental gene therapy. Two children were suffering from adenosine deaminase deficiency (ADA-SCID), a monogenetic disease leading to severe immunodeficiency. They were treated with white blood cells taken from their own blood (autologous therapy) and modified ex vivo to express the normal gene for making adenosine deaminase. After twelve years performing experimental protocols, Glybera (alipogene tiparvovec) became the first approved gene therapy, intended to treat lipoprotein lipase (LPL) deficiency, by European Medicines Agency in 2012 (Exhibit 1). However, Glybera was never approved by FDA in the U.S.
Exhibit 1. History of Gene Therapy
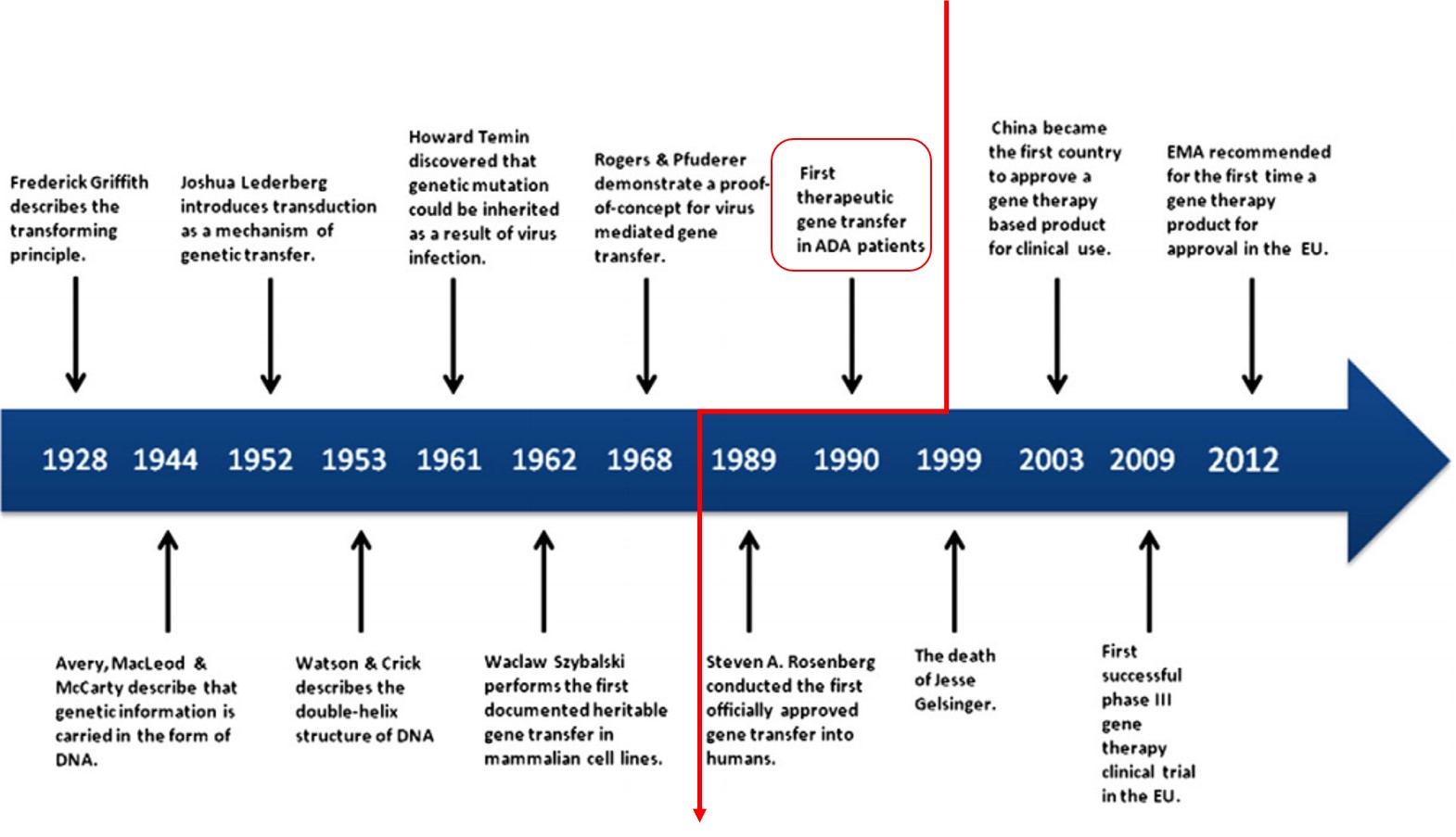
Source: T. Wirth et al., Gene 525 (2013)
162–169
Over the three decades since the first clinical trial, there have been many attempts using gene therapy for treatment of various indications. Among over 2 thousand clinical trials, the majority focused on cancer indications (65%, Exhibit 2). Despite many early failures, there is an increasing number of therapeutic successes in human clinical trials being reported in recent years. As a result, investments in gene therapy technologies are increasing rapidly. The disease targets of gene therapy are currently center around rare, inherited genetic diseases.
Exhibit 2. Various Indications Addressed by Gene
Therapy in Clinical Trials
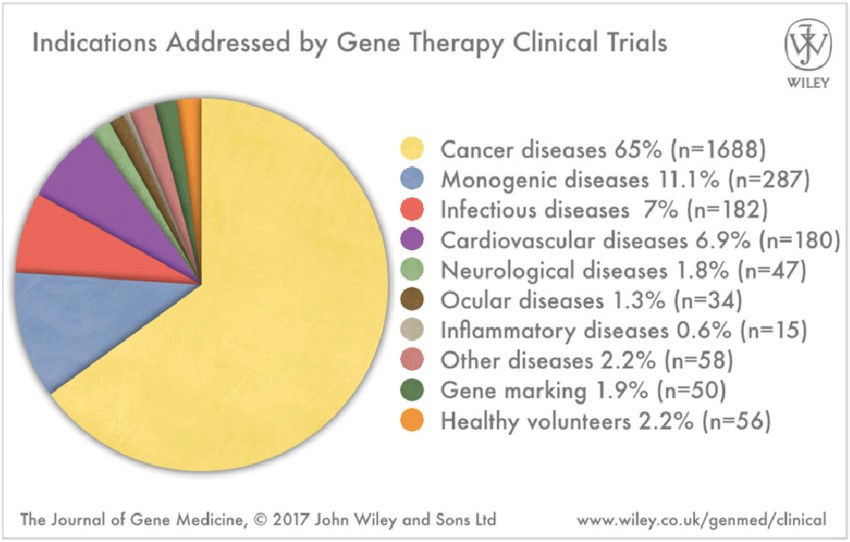
Source: The Journal of Gene Medicine,
2017, John Wiley and Sons Ltd.