|
|
||||||||
|
Odyssey Elixir CEO Scott Frohman provides a preview of their upcoming presentation at NobleCon18 NobleCon18 – Noble Capital Markets 18th Annual Small and Microcap Investor Conference – April 19-21, 2022 – Hard Rock, Hollywood, FL 100+ Public Company Presentations | Scheduled Breakouts | Panel Presentations | High-Profile Keynotes | Educational Sessions | Receptions & Networking Events Free Registration Available – More InfoMore info on Odyssey ElixirNobleCon18 Presenting Companies
About Odyssey Based in Fort Lauderdale, Florida, Odyssey Wellness LLC is an emerging, fast-growing RTD functional beverage company. Their innovative and exotic flavor-forward, functional mushroom elixirs are rich in active compounds found in the fruiting body of a mushrooms such as Shitake, Lion’s Mane, Reishi, Turkey Tail, Maitake, Chaga, and Cordyceps. These mushrooms have been revered throughout history as having medicinal qualities. |
||||||||
|
|
||||||||
|
Alvopetro Energy provides a preview of their upcoming presentation at NobleCon18 NobleCon18 – Noble Capital Markets 18th Annual Small and Microcap Investor Conference – April 19-21, 2022 – Hard Rock, Hollywood, FL 100+ Public Company Presentations | Scheduled Breakouts | Panel Presentations | High-Profile Keynotes | Educational Sessions | Receptions & Networking Events Free Registration Available – More InfoResearch News and Advanced Market Data on ALVOFNobleCon18 Presenting Companies Alvopetro Energy Ltd.’s vision is to become a leading independent upstream and midstream operator in Brazil. Our strategy is to unlock the on-shore natural gas potential in the state of Bahia in Brazil, building off the development of our Caburé natural gas field and our strategic midstream infrastructure. |
||||||||
|
|
|
GlobalX CFO Ryan Goepel provides a preview of their upcoming presentation at NobleCon18 NobleCon18 – Noble Capital Markets 18th Annual Small and Microcap Investor Conference – April 19-21, 2022 – Hard Rock, Hollywood, FL 100+ Public Company Presentations | Scheduled Breakouts | Panel Presentations | High-Profile Keynotes | Educational Sessions | Receptions & Networking Events Free Registration Available – More InfoNews and Advanced Market Data on JETMFNobleCon18 Presenting Companies
About GlobalX GlobalX is a US 121 domestic flag and supplemental airline flying the Airbus A320 family aircraft. GlobalX flies as an ACMI and charter airline serving the US, Caribbean, and Latin American markets. For more information, please visit www.globalxair.com . |
Schwazze (SHWZ) – Post Call Commentary and Updated Model

Monday, April 11, 2022
Schwazze (SHWZ)
Post Call Commentary and Updated Model
Medicine Man Technologies, Inc. is now operating under its new trade name, Schwazze. Schwazze is executing its strategy to become a leading vertically integrated cannabis holding company with a portfolio consisting of top-tier licensed brands spanning cultivation, extraction, infused-product manufacturing, dispensary operations, consulting, and a nutrient line. Schwazze leadership includes Colorado cannabis leaders with proven expertise in product and business development as well as top-tier executives from Fortune 500 companies. As a leading platform for vertical integration, Schwazze is strengthening the operational efficiency of the cannabis industry in Colorado and beyond, promoting sustainable growth and increased access to capital, while delivering best-quality service and products to the end consumer. The corporate entity continues to be named Medicine Man Technologies, Inc.
Joe Gomes, Senior Research Analyst, Noble Capital Markets, Inc.
Joshua Zoepfel, Research Associate, Noble Capital Markets, Inc.
Refer to the full report for the price target, fundamental analysis, and rating.
Post Management Call. We had an opportunity to speak with Schwazze CEO Justin Dye and CFO Nancy Huber following the release of fourth quarter earnings. Our discussion revolved around the Colorado and New Mexico operations, what’s next for those operations, and where the Company can go from here, including potential additional expansion.
Implementing the Playbook. All but one of the announced acquisitions has now been closed. We view 2022 as a year of integrating the acquisitions and implementing the Schwazze operating playbook to drive revenue and margins. While the cannabis market will see ebbs and flows, we expect Schwazze to…
This Company Sponsored Research is provided by Noble Capital Markets, Inc., a FINRA and S.E.C. registered broker-dealer (B/D).
*Analyst certification and important disclosures included in the full report. NOTE: investment decisions should not be based upon the content of this research summary. Proper due diligence is required before making any investment decision.
Tokens.com (SMURF) – Is This the Real Life or is it Fantasy?

Monday, April 11, 2022
Tokens.com (SMURF)
Is This the Real Life or is it Fantasy?
Tokens.com Corp is a Proof-of-Stake technology company that provides investors with a secure way to gain exposure to staking rewards and cryptocurrencies. It provides investors with exposure to the digital assets that power Decentralized Finance and Non-Fungible Tokens, without the burden of buying, managing, and securing digital assets themselves. The company creates value for its investors through earning Staking yields and the appreciation of its digital asset inventory, all achieved through environmentally friendly technology.
Joe Gomes, Senior Research Analyst, Noble Capital Markets, Inc.
Joshua Zoepfel, Research Associate, Noble Capital Markets, Inc.
Refer to the full report for the price target, fundamental analysis, and rating.
Taking Up More Space. Tokens.com’s management recently announced the acquisition of 40 plots of virtual land from SuperWorld. In this acquisition, Metaverse Group acquired landmark locations such as the Central Park Zoo, the Eden Fine Art Gallery in Manhattan, the Pelican Hotel in Miami Beach, and the Louis Vuitton store in Las Vegas.
What is SuperWorld? SuperWorld is a virtual world that has digitally mapped Planet Earth and plotted the land to be sold as non-fungible token (NFT) virtual real estate. Each plot of land corresponds to a physical world space, and includes landmarks such as…
This Company Sponsored Research is provided by Noble Capital Markets, Inc., a FINRA and S.E.C. registered broker-dealer (B/D).
*Analyst certification and important disclosures included in the full report. NOTE: investment decisions should not be based upon the content of this research summary. Proper due diligence is required before making any investment decision.
Release – Cypress Development Reports Water Rights Petition Dismissed
Cypress Development Reports Water Rights Petition Dismissed
Research, News, and Market Data on Cypress Development
April 11, 2022 – Vancouver, Canada – Cypress
Development Corp. (TSXV:
CYP) (OTCQX: CYDVF) (Frankfurt: C1Z1) ( “Cypress” or “the Company”) is pleased to report the Company has been informed that the petition for judicial review of the Nevada State Engineer’s extension of Water Right Permit 44411 and Certificate 13631 (the “Permit”) was dismissed with prejudice by the Fifth Judicial Court of Esmeralda County, Nevada.
The Company acquired the Permit from Intor Resources Corporation (“Intor”), a subsidiary of Nevada Sunrise Gold Corp., for use at the Company’s Clayton Valley Lithium Project, in Nevada (see news
release dated December 8, 2021). The Permit allows for the appropriation of the public waters of the State of Nevada in the amount of 1,770 acre-feet of groundwater per year for mining, milling and domestic use. This amount represents the largest single volume of permitted water available in the Clayton Valley, which is a fully appropriated hydrogeographic basin.
Intor submitted an Application for Extension of Time to Prevent Forfeiture of the Permit on July 29, 2021. The extension was approved by the State Engineer on November 2, 2021. With the court order, the State Engineer’s approval is final and binding, and the extension is valid until August 28, 2022.
About Cypress Development Corp
Cypress Development Corp. is a Canadian based advanced stage lithium company, focused on developing its 100%-owned Clayton Valley Lithium Project in Nevada, USA. Cypress is in the pilot stage of testing on material from its lithium-bearing claystone deposit and progressing towards completing a Feasibility Study and permitting, with the goal of becoming a domestic producer of lithium for the growing electric vehicle and battery storage market.
ON BEHALF OF CYPRESS DEVELOPMENT CORP.
WILLIAM WILLOUGHBY, PhD., PE
President & Chief Executive Officer
For further information, please contact:
Spiros Cacos | Vice President, Investor Relations
D: +1 604 764 1851 | Toll : 1 800 567 8181 | scacos@cypressdevelopmentcorp.com
www.cypressdevelopmentcorp.com
NEITHER THE TSX VENTURE EXCHANGE NOR ITS REGULATION SERVICES PROVIDER ACCEPTS RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THE CONTENT OF THIS NEWS RELEASE.
Cautionary Note Regarding Forward-Looking
Statements
This release includes certain statements that may be deemed
to be “forward-looking statements”. Forward-looking statements
are subject to risks, uncertainties and assumptions and are identified by words
such as “expects,”
“estimates,” “projects,” “anticipates,” “believes,” “could,” “scheduled,” and
other similar words. All statements in this release, other than statements
of historical facts, that address events or developments that management of the
Company expects, are forward-looking statements. Although management believes
the expectations expressed in such forward-looking statements are based on
reasonable assumptions, such statements are not guarantees of future
performance, and actual results or developments may differ materially from
those in the forward-looking statements. The Company undertakes no obligation
to update these forward-looking statements if management’s beliefs, estimates
or opinions, or other factors, should change. Factors that could cause actual
results to differ materially from those in forward-looking statements, include
market prices, exploration, and development successes, continued availability
of capital and financing, and general economic, market or business conditions.
Please see the public filings of the Company at www.sedar.com
Release – Comtech Welcomes Robert Samuels as Vice President of Investor Relations and Corporate Communications
Comtech Welcomes Robert Samuels as Vice President of Investor Relations and Corporate Communications
Research, News, and Market Data on Comtech Telecommunications
Samuels Takes IR Helm as Comtech Scales into Growing Failsafe
Communications Market Opportunities
Samuels brings over 20 years of
“As we turn
“I’ve focused my work on industries that impact people in their daily lives,” said
About
Certain information in this press release contains statements that are forward-looking in nature and involve certain significant risks and uncertainties. Actual results could differ materially from such forward-looking information. The Company’s
PCMTL
View source version on businesswire.com: https://www.businesswire.com/news/home/20220408005385/en/
Investor Relations
robert.samuels@comtech.com
Source:
Release – Endeavour Silver Delivers Strong Production in Q1 2022
Endeavour Silver Delivers Strong Production in Q1 2022
Research, News, and Market Data on Endeavour Silver
VANCOUVER, British
Columbia, April 11, 2022 (GLOBE NEWSWIRE) — Endeavour Silver Corp.
(“Endeavour” or the “Company”) (NYSE: EXK; TSX: EDR) is pleased to report first quarter 2022 production of 1,314,955 silver ounces (oz) and 8,695 gold oz, for silver equivalent 1 (“AgEq”) production of 2.0 million oz.
“The year is off to a strong start,” stated Dan Dickson, Chief Executive Officer. “Operationally, Guanacevi continues to outperform production expectations and Bolañitos remains steady. Strategically, we made a significant move in January signing a definitive agreement to acquire the Pitarrilla Project, one of the world’s largest undeveloped silver deposits. The addition of Pitarrilla, which is expected to close in the second quarter, significantly enhances our already attractive pipeline of growth projects, which also includes Terronera and Parral.”
Q1 2022
Highlights
- Guanacevi
Continued to Outperform: Silver and gold production exceeded plan driven by higher grades. - Bolañitos’
Performance Remained Steady: Strong silver production, higher silver grades and increased throughput were offset by the impact of lower than anticipated gold production and lower gold grades. - Metal
Sales and Inventories : Sold 1,717,768 oz silver and 8,381 oz gold during the quarter. Held 608,788 oz silver and 1,911 oz gold of bullion inventory and 59,594 oz silver and 1,931 oz gold in concentrate inventory at quarter end. - Advancing
the Terronera Project : Work continued on final detailed engineering, early earth works, critical contracts and the procurement of long lead items. The Company intends to make a formal construction decision subject to completion of a financing package and receipt of additional amended permits in the coming months. - Announced
Definitive Agreement to Acquire the Pitarrilla Project: Endeavour is acquiring Pitarrilla, one of the largest undeveloped silver deposits in the world, from SSR Mining Inc. in a transaction expected to close in Q2 2022. Pitarrilla is located in Durango State, Mexico, which has a long history of mining and is known as a mining-friendly jurisdiction with several mines in operation, including our Guanacevi mine. - Completed
US$46.0 Million Bought Deal Financing: On March 22, 2022 Endeavour completed a prospectus offering for the issuance of 9,293,150 common shares at a price of US$4.95 per common share for gross proceeds of US$46.0 million, including the exercise of an over-allotment option. The Company plans to use the net proceeds to pay the US$35 million cash consideration payable to SSR Mining Inc. on completion of the Company’s acquisition of the Pitarrilla project and for the Company’s general corporate purposes and working capital.
Q1 2022 Mine
Operations
Consolidated silver production increased by 25% to 1,314,955 ounces in Q1 2022 compared to Q1 2021, primarily driven by a 23% increase in silver production at the Guanacevi mine and a 70% increase in silver production at the Bolañitos mine offset by nil production at El Compas, which the Company put on care and maintenance last August.
Gold production decreased by 22% to 8,695 ounces as a 27% increase in gold production at the Guanacevi mine was offset by a 16% decrease in gold production at the Bolañitos mine and nil production at El Compas.
Guanacevi throughput in Q1 2022 was 14% higher than Q1 2021 and silver grades and gold grades were 10% and 13% higher, respectively. Guanacevi throughput met plan and mining the new higher grade El Curso orebody has led to significantly improved grades and mine flexibility. Additionally, supplies of local third-party ores continued to supplement mine production, amounting to 11% of quarterly throughput and contributing to the higher ore grades.
Bolañitos Q1 2022 throughput was 7% higher than Q1 2021 with silver grades 61% higher and gold grades 20% lower. Silver production increased by 70% while gold production decreased by 16% at the Bolañitos mine.
Production
Highlights for the Three Months Ended March 31, 2022
|
Q1 |
Three Months Ended March 31, |
||
|
|
2022 |
2021 |
% Change |
|
Throughput (tonnes) |
206,147 |
209,453 |
(2%) |
|
Silver ounces produced |
1,314,955 |
1,048,100 |
25% |
|
Gold ounces produced |
8,695 |
11,109 |
(22%) |
|
Payable silver ounces produced |
1,303,540 |
1,036,710 |
26% |
|
Payable gold ounces produced |
8,549 |
10,894 |
(22%) |
|
Silver equivalent ounces produced 1 |
2,010,555 |
1,936,820 |
4% |
|
Silver ounces sold |
1,717,768 |
623,379 |
176% |
|
Gold ounces sold |
8,381 |
10,663 |
(21%) |
Q1 2022
Production by Mine
|
Production |
Tonnes |
Tonnes |
Grade |
Grade |
Recovery |
Recovery |
Silver |
Gold |
|
by |
Produced |
per day |
Ag gpt* |
Au gpt* |
Ag % |
Au % |
Oz |
Oz |
|
Guanaceví |
101,253 |
1,125 |
407 |
1.19 |
85.6% |
89.8% |
1,133,850 |
3,477 |
|
Bolañitos |
104,894 |
1,165 |
61 |
1.73 |
88.0% |
89.4% |
181,105 |
5,218 |
|
Consolidated |
206,147 |
2,291 |
231 |
1.46 |
85.9% |
89.6% |
1,314,955 |
8,695 |
*gpt = grams per
tonne
Q1 2022 Financial
Results and Conference Call
The Company’s Q1 2022 financial results will be released before markets open on Wednesday, May 11, 2022 and a telephone conference call will be held the same day at 10:00 a.m. PT / 1:00 p.m. ET. To participate in the conference call, please dial the numbers below.
|
Date & Time: |
Wednesday, May 11, 2022 at 10:00 a.m. PT / 1:00 p.m. ET |
|
|
|
|
Telephone: |
Toll-free in Canada and the US +1-800-319-4610 |
|
|
Local or International +1-604-638-5340 |
|
|
Please allow up to 10 minutes to be connected to the conference call. |
|
|
|
|
Replay: |
A replay of the conference call will be available by dialing (toll-free) +1-800-319-6413 in Canada and the US (toll-free) or +1-604-638-9010 outside of Canada and the US. The replay passcode is 8312#. The replay will also be available on the Company’s website at www.edrsilver.com |
About Endeavour
Silver – Endeavour Silver Corp. is a mid-tier precious metals mining company that operates two high-grade underground silver-gold mines in Mexico. Endeavour is currently advancing the Terronera mine project towards a development decision, pending financing and final permits and exploring its portfolio of exploration and development projects in Mexico, Chile and the United States to facilitate its goal to become a premier senior silver producer. Our philosophy of corporate social integrity creates value for all stakeholders.
SOURCE Endeavour Silver Corp.
Contact
Information
Trish Moran
Interim Head of Investor Relations
Tel: (416) 564-4290
Email: pmoran@edrsilver.com
Website: www.edrsilver.com
Follow Endeavour Silver on Facebook ,
Twitter ,
Instagram and
LinkedIn
Cautionary Note
Regarding Forward-Looking Statements
This news release
contains “forward-looking statements” within the meaning of the United States
private securities litigation reform act of 1995 and “forward-looking
information” within the meaning of applicable Canadian securities legislation.
Such forward-looking statements and information herein include but are not
limited to statements regarding Endeavour’s anticipated performance in 2022
including changes in mining operations and production levels, the timing and
results of various activities and the impact of the COVID 19 pandemic on
operations. The Company does not intend to and does not assume any obligation
to update such forward-looking statements or information, other than as
required by applicable law.
Forward-looking
statements or information involve known and unknown risks, uncertainties and
other factors that may cause the actual results, level of activity, production
levels, performance or achievements of Endeavour and its operations to be
materially different from those expressed or implied by such statements. Such
factors include but are not limited to the ultimate impact of the COVID 19
pandemic on operations and results, changes in production and costs guidance,
national and local governments, legislation, taxation, controls, regulations
and political or economic developments in Canada and Mexico; financial risks
due to precious metals prices, operating or technical difficulties in mineral exploration,
development and mining activities; risks and hazards of mineral exploration,
development and mining; the speculative nature of mineral exploration and
development, risks in obtaining necessary licenses and permits, and challenges
to the Company’s title to properties; as well as those factors described in the
section “risk factors” contained in the Company’s most recent form 40F/Annual
Information Form filed with the S.E.C. and Canadian securities regulatory
authorities.
Forward-looking
statements are based on assumptions management believes to be reasonable,
including but not limited to: the continued operation of the Company’s mining
operations, no material adverse change in the market price of commodities,
mining operations will operate and the mining products will be completed in
accordance with management’s expectations and achieve their stated production
outcomes, and such other assumptions and factors as set out herein. Although
the Company has attempted to identify important factors that could cause actual
results to differ materially from those contained in forward-looking statements
or information, there may be other factors that cause results to be materially
different from those anticipated, described, estimated, assessed or intended. There
can be no assurance that any forward-looking statements or information will
prove to be accurate as actual results and future events could differ
materially from those anticipated in such statements or information.
Accordingly, readers should not place undue reliance on forward-looking
statements or information.
_____________________________________________
1 Silver equivalent calculated using an 80:1 silver:gold ratio.
Release – Lineage to Present at the NobleCon18 Investor Conference on April 20, 2022
Lineage to Present at the NobleCon18 Investor Conference on April 20, 2022
Research, News, and Market Data on Lineage Cell Therapeutics
CARLSBAD, Calif.–(BUSINESS WIRE)–Apr. 11, 2022–
(NYSE American and TASE: LCTX), a clinical-stage biotechnology company developing allogeneic cell therapies for unmet medical needs, today announced that
in Seminole Ballroom A. NobleCon18 is taking place at the
An archived webcast of the corporate presentation will be available starting
About
Cell Therapeutics, Inc.
View source version on businesswire.com:
https://www.businesswire.com/news/home/20220411005035/en/
ir@lineagecell.com)
(442) 287-8963
Solebury
Trout IR
Mbiega@soleburytrout.com)
(617) 221-9660
Partners
David.schull@russopartnersllc.com
(212) 845-4242
Source:
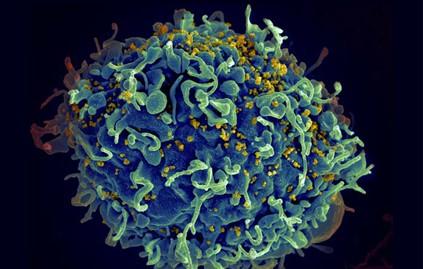
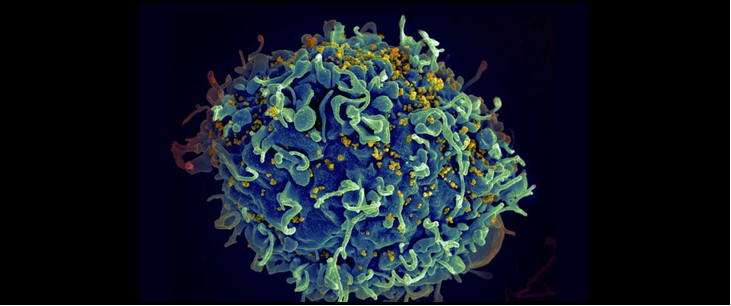
New Tool Reveals How Immune Cells Find their Targets
Image credit: S. Pincus, E. Fischer, Austin Athman (NIH)
Improved Method to Identify B or T cells that Interact with Viral or Bacterial Proteins
Anne Trafton | MIT News Office
The human body has millions of unique B and T cells that roam the body, looking for microbial invaders. These immune cells’ ability to recognize harmful microbes is critical to successfully fighting off infection.
MIT biological engineers have now devised an experimental tool that allows them to precisely pick out interactions between a particular immune cell and its target antigen. The new technique, which uses engineered viruses to present many different antigens to huge populations of immune cells, could allow large-scale screens of such interactions.
“This technique leads the way to understand immunity much closer to how the immune system itself actually works, will help researchers make sense of complex immune recognition in a variety of diseases, and could accelerate the development of more effective vaccines and immunotherapies,” says Michael Birnbaum, an associate professor of biological engineering at MIT, a member of MIT’s Koch Institute for Integrative Cancer Research, and the senior author of the study.
Former MIT graduate student Connor Dobson is the lead author of the paper, which appears in Nature Methods.
A Simple Screen for a Complex System
Both B and T cells play critical roles in launching an immune response. When a T cell encounters its target, it starts proliferating to produce an army of identical cells that can attack infected cells. And B cells that encounter their target begin producing antibodies that help recruit other components of the immune system to clear the infection.
Scientists who study the immune system have several tools to help them identify specific antigen-immune cell interactions. However, these tools generally only allow for the study of a large pool of antigens exposed to one B or T cell, or a large pool of immune cells encountering a small number of antigens.
“In your body, you have millions of unique T cells, and they could recognize billions of possible antigens. All of the tools that have been developed to this point are really designed to look at one side of that diversity at a time,” Birnbaum says.
The MIT team set out to design a new tool that would let them screen huge libraries of both antigens and immune cells at the same time, allowing them to pick out any specific interactions within the vast realm of possibilities.
To create a simple way to screen so many possible interactions, the researchers engineered a specialized form of a lentivirus, a type of virus that scientists often use to deliver genes because it can integrate pieces of DNA into host cells. These viruses have an envelope protein called VSV-G that can bind to receptors on the surface of many types of human cells, including immune cells, and infect them.
For this study, the researchers modified the VSV-G protein so that it cannot infect a cell on its own, instead relying on an antigen of the researchers’ choosing. This modified version of VSV-G can only help the lentivirus get into a cell if the paired antigen binds to a human B or T-cell receptor that recognizes the antigen.
Once the virus enters, it integrates itself into the host cell’s genome. Therefore, by sequencing the genome of all the cells in the sample, the researchers can discover both the antigen expressed by the virus that infected the cell and the sequence of the T or B-cell receptor that allowed it to enter.
“In this way, we can use viral infection itself as a way to match up and then identify antigen-immune cell parings,” Birnbaum says.
Interactions Identified
To demonstrate the accuracy of their technique, the researchers created a pool of viruses with antigens from 100 different viruses, including influenza, cytomegalovirus, and Epstein-Barr virus. They screened these viruses against about 400,000 T cells and showed that the technique could correctly pick out antigen-T-cell receptor pairings that had been previously identified.
The researchers also screened two different B-cell receptors against 43 antigens, including antigens from HIV and the spike protein of SARS-CoV-2.
In future studies, Birnbaum hopes to screen thousands of antigens against B and T cell populations. “Our ideal would be to screen entire viruses or families of viruses, to be able to get a readout of your entire immune system in one experiment,” he says.
In one study that is now ongoing, Birnbaum’s lab is working with researchers at the Ragon Institute of MGH, MIT, and Harvard to study how different people’s immune systems respond to viruses such as HIV and SARS-CoV-2. Such studies could help to reveal why some people naturally fight off certain viruses better than others, and potentially lead to the development of more effective vaccines.
The researchers envision that this technology could also have other uses. Birnbaum’s lab is now working on adapting the same viruses to deliver engineered genes to target cells. In that case, the viruses would carry not only a targeting molecule but also a novel gene that would be incorporated exclusively into cells that have the right target. This could offer a way to selectively deliver genes that promote cell death into cancer cells, for example.
“We built this tool to look for antigens, but there’s nothing particularly special about antigens,” Birnbaum says. “You could potentially use it to go into specific cells in order to do gene modifications for cell and gene therapy.”
Suggested Reading
 Stem-Cell Based Therapy for Alzheimer’s Disease
|
 Stem Cells Role in the Anti-Aging Business
|
 Stem Cell-Derived Retinal Pigment Epithelium Cells – Vision for the Future
|
 The Appeal of EVs with Bidirectional Charging
|
Stay up to date. Follow us:

|
Lithium Inflation and Availability Concerns Elon Musk
Image credit: Steve Jurvetson (Flickr)
Elon Musk Does Not Want Lithium Production Levels to Stall the Growth of Tesla
Is the pace of lithium extraction going to continue to be a drag on EV manufacturing? Elon Musk, CEO of Tesla (TSLA), has no intention of allowing lithium costs or availability to depress his EV company’s growth. In one of his more serious tweets, Musk mentions the challenge of securing supply for Lithium-ion batteries and hints at a possible solution for the company he founded.
Musk was commenting on a Tweet from World of Statistics, which showed the price of lithium over the past ten years has risen from $4,450/tonne to $78,032/tonne today. Musk’s tweet offered a possible solution for Tesla to get ahead of the supply issue (a tonne, or metric Ton, is equal to 1000kg, about 2,205 pounds).
Musk’s Lithium Comment
In his tweet he mentions how “insane” the price of lithium has become. He also points out that the world has enough of the mineral, but extracting and refining hasn’t been able to keep up with growing needs. The CEO even suggested that Tesla might get into the mining business to help resolve shortages of the critical element used in li-ion batteries.
If Tesla also mined and refined needed raw materials to make sure production growth doesn’t stall, it would be unique in car company history. And, from an investor’s standpoint, it opens questions. We recently experienced how the chip shortage created havoc for car companies and buyers. One missing ingredient can prevent manufacturing at the ideal pace to meet consumer demand. Will locking up long-term lithium supply become standard? Will Tesla and other auto companies integrate supplying themselves with materials become part of who survives in the EV era and who doesn’t? What does this mean for li-ion-related stocks?
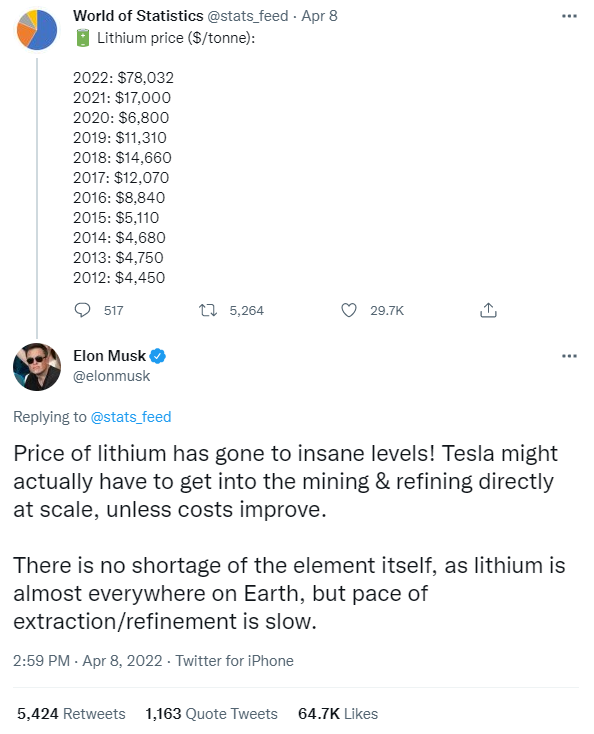
“Insane Levels”
Benchmark lithium prices are at about $78,000/tonne; this is an 80% increase from January 1st. Barrons tracks the price for a basket of lithium battery elements and has reported that it is up 60% on the year (April 2022). This increase adds on average another $2,000 to the price of an EV. Most materials to manufacture batteries (copper, cobalt, nickel, lithium) are secured on a contract basis. This helps smooth unexected changes allowing companies to better predict future costs.
Looking Forward
In a later tweet Musk wrote, “we have some cool ideas for sustainable lithium extraction [and] refinement.” Which will be worth watching. In the interim, lithium mining stocks are up 20-30% this year. Many are playing catch-up and and building their capacity to meet EV demand growth. The miners are behind the growth curve, but that isn’t because of a shortage of the mineral.
Managing Editor, Channelchek
Suggested Reading
 Lithium Prices Continue Their Ascent
|
 Enough US Produced Lithium to Exceed Today’s Demand
|
 Lithium-Ion Power vs Hydrogen Fuel Cell
|
 Michael Burry Sees Positive in Elon Musk’s Twitter Stake
|
Sources
https://www.teslarati.com/tesla-lithium-mining/
Stay up to date. Follow us:

|
BlackBoxStocks (BLBX) Scheduled to Present at NobleCon18 Investor Conference
|
|
|
BlackBoxStocks provides a preview of their upcoming presentation at NobleCon18 NobleCon18 – Noble Capital Markets 18th Annual Small and Microcap Investor Conference – April 19-21, 2022 – Hard Rock, Hollywood, FL 100+ Public Company Presentations | Scheduled Breakouts | Panel Presentations | High-Profile Keynotes | Educational Sessions | Receptions & Networking Events Free Registration Available – More InfoResearch News and Advanced Market Data on BLBXNobleCon18 Presenting Companies
About Blackboxstocks Blackboxstocks, Inc. is a financial technology and social media hybrid platform offering real-time proprietary analytics and news for stock and options traders of all levels. Our web-based software employs “predictive technology” enhanced by artificial intelligence to find volatility and unusual market activity that may result in the rapid change in the price of a stock or option. Blackbox continuously scans the NASDAQ, New York Stock Exchange, CBOE, and all other options markets, analyzing over 10,000 stocks and up to 1,500,000 options contracts multiple times per second. We provide our users with a fully interactive social media platform that is integrated into our dashboard, enabling our users to exchange information and ideas quickly and efficiently through a common network. We recently introduced a live audio/video feature that allows our members to broadcast on their own channels to share trade strategies and market insight within the Blackbox community. Blackbox is a SaaS company with a growing base of users that spans 42 countries; current subscription fees are $99.97 per month or $959.00 annually. For more information, go to: www.blackboxstocks.com . |
Lineage Cell Therapeutics (LCTX) Scheduled to Present at NobleCon18 Investor Conference
|
|
|
Lineage Cell Therapeutics CEO Brian Culley provides a preview of their upcoming presentation at NobleCon18 NobleCon18 – Noble Capital Markets 18th Annual Small and Microcap Investor Conference – April 19-21, 2022 – Hard Rock, Hollywood, FL 100+ Public Company Presentations | Scheduled Breakouts | Panel Presentations | High-Profile Keynotes | Educational Sessions | Receptions & Networking Events Free Registration Available – More InfoResearch News and Advanced Market Data on LCTXNobleCon18 Presenting Companies
About Lineage Cell Therapeutics Lineage Cell Therapeutics is a clinical-stage biotechnology company developing novel cell therapies for unmet medical needs. Lineage’s programs are based on its robust proprietary cell-based therapy platform and associated in-house development and manufacturing capabilities. With this platform Lineage develops and manufactures specialized, terminally differentiated human cells from its pluripotent and progenitor cell starting materials. These differentiated cells are developed to either replace or support cells that are dysfunctional or absent due to degenerative disease or traumatic injury or administered as a means of helping the body mount an effective immune response to cancer. Lineage’s clinical programs are in markets with billion dollar opportunities and include four allogeneic (“off-the-shelf”) product candidates: (i) OpRegen, a retinal pigment epithelium transplant therapy in Phase 1/2a development for the treatment of dry age-related macular degeneration, which is now being developed under a worldwide collaboration with Roche and Genentech, a member of the Roche Group; (ii) OPC1, an oligodendrocyte progenitor cell therapy in Phase 1/2a development for the treatment of acute spinal cord injuries; (iii) VAC2, a dendritic cell therapy produced from Lineage’s VAC technology platform for immuno-oncology and infectious disease, currently in Phase 1 clinical development for the treatment of non-small cell lung cancer and (iv) ANP1, an auditory neuronal progenitor cell therapy for the potential treatment of auditory neuropathy. For more information, please visit www.lineagecell.com or follow the Company on Twitter @LineageCell. |