Comtech Telecommunications (CMTL) Corporate Presentation from NobleCon18Research, News and Market Data on Comtech TelecommunicationsNobleCon 18 Complete Rebroadcast
|
Category:
Cumulus Media (CMLS) NobleCon18 Presentation Replay
Release – Bowlero Corp. to Report Financial Results for the Third Quarter of Fiscal Year 2022
Bowlero Corp. to Report Financial Results for the Third Quarter of Fiscal Year 2022
Research, News, and Market Data on Bowlero
RICHMOND, Va., May 02, 2022 (GLOBE NEWSWIRE) — Bowlero Corp. (NYSE: BOWL) (“Bowlero” or the “Company”), the world’s largest owner and operator of bowling centers, will report financial results for the third quarter of fiscal year 2022 on Wednesday May 11, 2022 after the market closes.
Listeners may access an investor webcast hosted by Bowlero. The webcast and results presentation will be accessible Wednesday May 11, 2022 at 5:30 PM ET in the Events & Presentations section of the Bowlero Investor Relations website at
https://ir.bowlerocorp.com/overview/default.aspx.
About
Bowlero Corp.
Bowlero Corp. is the worldwide leader in bowling entertainment, media, and events. With more than 300 bowling centers across North America, Bowlero Corp. serves more than 26 million guests each year through a family of brands that includes Bowlero, Bowlmor Lanes, and AMF. In 2019, Bowlero Corp. acquired the Professional Bowlers Association, the major league of bowling, which boasts thousands of members and millions of fans across the globe. For more information on Bowlero Corp., please visit BowleroCorp.com.
Contacts:
For Media:
ICR, Inc.
Tom Vogel
Tom.Vogel@icrinc.com
For Investors:
ICR, Inc.
Ryan Lawrence
Ryan.Lawrence@icrinc.com
Ashley DeSimone
Ashley.desimone@icrinc.com
Source: Bowlero Corp
Release – Aurania Appoints Senior Geological Consultant, Dr. Cristian Vallejo
Aurania Appoints Senior Geological Consultant, Dr. Cristian Vallejo
Research, News, and Market Data on Aurania Resources
Toronto, Ontario, May 2, 2022 –
Aurania Resources Ltd. (TSXV: ARU) (OTCQB: AUIAF) (Frankfurt: 20Q) (“Aurania”
or the “Company”) announces it has retained Dr. Cristian Vallejo of Geostrat S.A. as a Geological Consultant. Dr. Vallejo is currently working in the Patuca area of Aurania’s Lost Cities-Cutucu project (the “Project”) in southeastern Ecuador. The village of Patuca is located near a small alluvial gold mine which is just on the edge and outside the Company’s concessions. This small gold mine has been actively worked since at least 2008 when it was initially examined by Aurania’s President and CEO, Dr. Keith Barron and Professor Octavio Latorre.
Dr. Keith Barron, commented, “Because of the proximity of Aurania’s Crunchy Hill, Latorre, and Yawi epithermal prospects, I believe that the gold coming from the small alluvial gold mine near Patuca has its ultimate origin from the Aurania concessions. Cristian Vallejo has been retained to provide us guidance in this respect, using his knowledge of sedimentology.”
Dr. Vallejo is an Ecuadorian national and a Geologist with twenty-two years experience in the mining/oil industry and academia. During his PhD at the Swiss Federal Institute of Technology (ETH-Zurich) he worked on the geology of the Oriente Basin of Ecuador and the geodynamic evolution of the Western Cordillera of Ecuador and Colombia. In these studies, he applied field mapping, sedimentology, organic matter analysis, radiometric dating of igneous rocks, isotope geochemistry and provenance analysis.
After finishing his PhD he worked for two years as Project Manager of Salazar Resources in the Curipamba Project, being part of the team that discovered the El Domo Volcanogenic Massive Sulphide deposit (VMS), currently in the mine construction phase.
During the last twelve years he has worked as a consultant for the oil industry in the geological modelling of the main oil fields of the Oriente Basin of Ecuador, Putumayo, Guajira and Magdalena basins of Colombia and the Peten Basin of Guatemala. The results of his studies have been published in peer-reviewed journals and presented at international geological meetings.
Most recently Cristian was lead author on: “Jurassic to Early Paleogene sedimentation in the Amazon region of Ecuador: Implications for the paleogeographic evolution of northwestern South America” published in September 2021. This work specifically refers to geological mapping he carried out in the Cordillera de Cutucu, within the confines of Aurania’s Lost Cities Project.
Dr. Barron further commented, “Back in 2008 there was only a single small miner’s concession at Patuca where they were recovering gold by hydrauliking hillsides and catching the gold in wooden sluice boxes. It was a very primitive operation, but it supported about a dozen people. What impressed me at the time was that this was clearly not a modern placer, but an ancient “paleoplacer” of unknown age. The source of the gold was not apparent, neither was there a stream or river nearby, though the deposit indicated that a substantial river must have existed there at one time. More recently, there has been what can only be described as an “explosion” of small miner activity and the majority of flowing streams in the Patuca area have now been staked, but this is a “red herring”. From a geological perspective, the miners may not appreciate that the gold occurrence, worked since 2008, is a fossil system. They are however recovering small amounts of “second or third cycle” gold; in other words, capturing gold from erosion of this paleoplacer and others nearby.
On a greater scale however, I believe Cristian is Ecuador’s authority on the Cutucu Basin, which contains Aurania’s copper-silver and zinc-silver-lead prospects hosted in sediments at Tsenken and Tiria-Shimpia. Due to his enormous experience in stratigraphy and sedimentology in the area he will be a valuable addition to Aurania’s team, and I believe will have much to contribute on these exciting prospects. For now, he will be examining and evaluating all of Aurania’s information collected to date.”
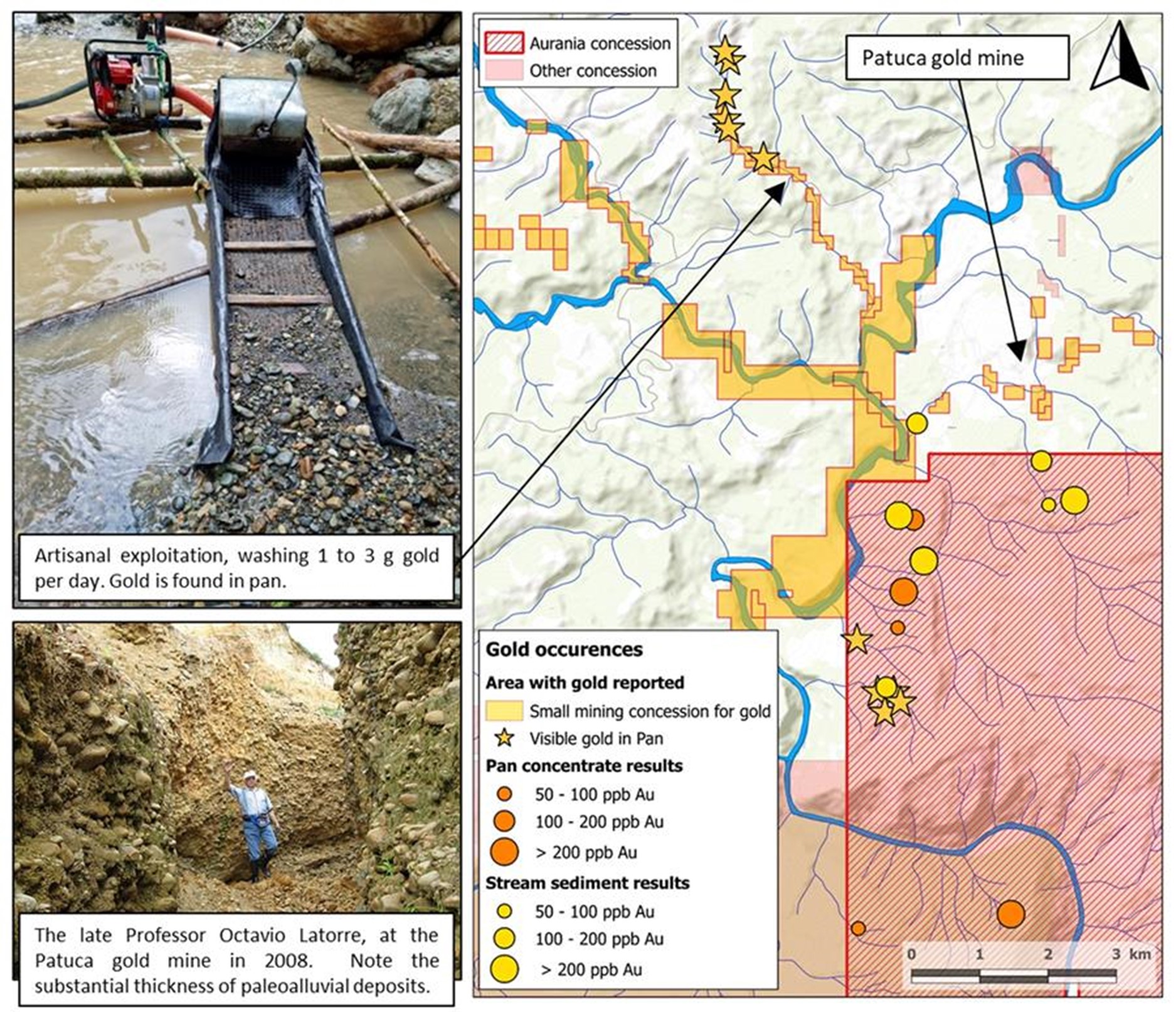
Figure 1: Gold results from the Aurania property near the artisanal Patuca Gold Mine.
Qualified Person
The geological information contained in this news release has been verified and approved by Jean-Paul Pallier, MSc. Mr. Pallier is a designated EurGeol by the European Federation of Geologists and a Qualified Person as defined by National Instrument 43-101, Standards of Disclosure for Mineral Projects of the Canadian Securities Administrators.
About Aurania
Aurania is a mineral exploration company engaged in the identification, evaluation, acquisition and exploration of mineral property interests, with a focus on precious metals and copper in South America. Its flagship asset, The Lost Cities – Cutucu Project, is located in the Jurassic Metallogenic Belt in the eastern foothills of the Andes mountain range of southeastern Ecuador.
Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward-Looking Statements
This news release may contain forward-looking information that involves substantial known and unknown risks and uncertainties, most of which are beyond the control of Aurania. Forward-looking statements include estimates and statements that describe Aurania’s future plans, objectives or goals, including words to the effect that Aurania or its management expects a stated condition or result to occur. Forward-looking statements may be identified by such terms as “believes”, “anticipates”, “expects”, “estimates”, “may”, “could”, “would”, “will”, or “plan”. Since forward-looking statements are based on assumptions and address future events and conditions, by their very nature they involve inherent risks and uncertainties. Although these statements are based on information currently available to Aurania, Aurania provides no assurance that actual results will meet management’s expectations. Risks, uncertainties and other factors involved with forward-looking information could cause actual events, results, performance, prospects and opportunities to differ materially from those expressed or implied by such forward-looking information. Forward looking information in this news release includes, but is not limited to Aurania’s objectives, goals or future plans, statements, exploration results, potential mineralization, the corporation’s portfolio, treasury, management team and enhanced capital markets profile, the estimation of mineral resources, exploration, timing of the commencement of operations and estimates of market conditions. Factors that could cause actual results to differ materially from such forward-looking information include, but are not limited to, failure to identify mineral resources, failure to convert estimated mineral resources to reserves, the inability to complete a feasibility study which recommends a production decision, the preliminary nature of metallurgical test results, delays in obtaining or failures to obtain required governmental, regulatory, environmental or other project approvals, political risks, inability to fulfill the duty to accommodate indigenous peoples, uncertainties relating to the availability and costs of financing needed in the future, changes in equity markets, inflation, changes in exchange rates, fluctuations in commodity prices, delays in the development of projects, capital and operating costs varying significantly from estimates and the other risks involved in the mineral exploration and development industry, the effects of COVID-19 on the business of the Company including but not limited to the effects of COVID-19 on the price of commodities, capital market conditions, restrictions on labour and international travel and supply chains, and those risks set out in Aurania’s public documents filed on SEDAR. Although Aurania believes that the assumptions and factors used in preparing the forward-looking information in this news release are reasonable, undue reliance should not be placed on such information, which only applies as of the date of this news release, and no assurance can be given that such events will occur in the disclosed time frames or at all. Aurania disclaims any intention or obligation to update or revise any forward-looking information, whether as a result of new information, future events or otherwise, other than as required by law.
Release – Ocugen to Host Conference Call on Friday, May 6 at 8:30 a.m. ET to Provide Business Update and Discuss First Quarter 2022 Financial Results
Ocugen to Host Conference Call on Friday, May 6 at 8:30 a.m. ET to Provide Business Update and Discuss First Quarter 2022 Financial Results
Research, News, and Market Data on Ocugen
MALVERN, Pa., May 02, 2022 (GLOBE NEWSWIRE) — Ocugen, Inc. (NASDAQ: OCGN), a biotechnology company focused on discovering, developing and commercializing novel gene therapies, biologicals and vaccines, today announced that it will host a conference call to provide a business update and discuss its first quarter 2022 financial results at 8:30 a.m. ET on Friday, May 6, 2022.
Ocugen will issue a pre-market earnings announcement on the same day. Investors are invited to participate on the call using the following details:
- Dial-In Number: (844) 873-7330 (toll free) or (602) 563-8473 (Int’l)
- Conference ID: 6995784
- Webcast: Available in the “Investors” section of the
Ocugen website and archived for approximately 45 days following the call
About Ocugen, Inc.
Ocugen, Inc. is a biotechnology company focused on discovering, developing, and commercializing novel gene therapies, biologicals and vaccines that improve health and offer hope for people and global communities. We are making an impact through courageous innovation, taking science in new directions in service of patients. Our breakthrough modifier gene therapy platform has the potential to treat multiple diseases with one drug and we are advancing research in other therapeutic areas to offer new options for people with unmet medical needs. Discover more at www.ocugen.com and follow us on Twitter and LinkedIn.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995, which are subject to risks and uncertainties. Such forward-looking statements within this press release include, without limitation, the intended use of net proceeds from the registered direct offering. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from our current expectations, such as market and other conditions. These and other risks and uncertainties are more fully described in our periodic filings with the Securities and Exchange Commission (the “SEC”), including the risk factors described in the section entitled “Risk Factors” in the quarterly and annual reports that we file with the SEC. Any forward-looking statements that we make in this press release speak only as of the date of this press release. Except as required by law, we assume no obligation to update forward-looking statements contained in this press release whether as a result of new information, future events or otherwise, after the date of this press release.
Ocugen Contact:
Ken Inchausti
Head, Investor Relations & Communications
ken.inchausti@ocugen.com
Please submit investor-related inquiries to:
IR@ocugen.com
Release – Alliance Resource Partners, L.P. Reports Increased Financial and Operating Results
Alliance Resource Partners, L.P. Increases Quarterly Cash Distribution 40% to $0.35 Per Unit; Provides Preliminary View of Results for the Quarter Ended March 31, 2022; and Increases Guidance
Research, News, and Market Data on Alliance Resource Partners
TULSA, Okla.–(BUSINESS WIRE)– Alliance Resource Partners, L.P. (NASDAQ: ARLP) today reported increased financial and operating results for the quarter ended March 31, 2022 (the “2022 Quarter”). Total revenues in the 2022 Quarter increased 44.6% to $460.9 million compared to $318.6 million for the quarter ended March 31, 2021 (the “2021 Quarter”) as a result of higher coal sales volumes and prices, which rose 19.5% and 13.0%, respectively, and higher oil & gas royalty volumes and prices, which increased by 26.3% and 74.9%, respectively. Total operating expenses increased to $373.0 million in the 2022 Quarter, compared to $282.3 million in the 2021 Quarter, due primarily to increased coal sales volumes and inflationary cost pressures. Income before income taxes increased 221.0% to $79.7 million in the 2022 Quarter as compared to $24.8 million in the 2021 Quarter. As previously reported, during the 2022 Quarter, ARLP recognized a one-time non-cash deferred income tax charge of $37.3 million and a current income tax expense of $4.8 million associated with its election to have our oil & gas royalty activities treated as a taxable entity for federal and state income tax purposes, which collectively reduced net income by $0.33 per basic and diluted limited partner unit. This election effectively reduces the total income tax burden on our oil & gas royalties, as ARLP will pay entity-level taxes at corporate tax rates that are well below the individual tax rates that would otherwise be paid by our unitholders. Reflecting higher revenues, partially offset by increased total operating and income tax expenses, net income for the 2022 Quarter increased to $36.7 million, or $0.28 per basic and diluted limited partner unit, compared to $24.7 million, or $0.19 per basic and diluted limited partner unit, for the 2021 Quarter. EBITDA also increased 61.5% in the 2022 Quarter to $152.3 million compared to $94.3 million in the 2021 Quarter. (Unless otherwise noted, all references in the text of this release to “net income” refer to “net income attributable to ARLP.” For a definition of EBITDA and related reconciliation to its comparable GAAP financial measure throughout this release, please see the end of this release.)
Compared to the quarter ended December 31, 2021 (the “Sequential Quarter”), total revenues decreased by 2.7% primarily as a result of lower coal sales volumes due to previously reported coal shipment delays, partially offset by higher coal sales price realizations and increased oil & gas royalty volumes and prices. Total operating expenses decreased 9.5% to $373.0 million due primarily to lower coal sales volumes in the 2022 Quarter and expenses incurred in the Sequential Quarter related to an $11.8 million buy-out of a coal contract and $6.8 million of unfavorable year end non-cash actuarial and accrual adjustments. Lower total operating expenses more than offset reduced revenues leading income before income taxes higher by 52.5% to $79.7 million in the 2022 Quarter compared to $52.2 million for the Sequential Quarter. Although income before income taxes was higher in the 2022 Quarter, net income decreased to $36.7 million compared to $51.8 million in the Sequential Quarter due to the impact of income tax expense attributable to the above discussed change in tax status of our oil & gas royalty segment. EBITDA increased 16.9% in the 2022 Quarter to $152.3 million compared to $130.2 million in the Sequential Quarter.
As previously announced on April 26, 2022, the Board of Directors of ARLP’s general partner (the “Board”) increased the cash distribution to unitholders for the 2022 Quarter to $0.35 per unit (an annualized rate of $1.40 per unit), payable on May 13, 2022, to all unitholders of record as of the close of trading on May 6, 2022. The announced distribution represents a 250.0% increase over the cash distribution of $0.10 per unit for the 2021 Quarter and a 40.0% increase over the cash distribution of $0.25 per unit for the Sequential Quarter.
“Buoyed by robust energy market fundamentals during the 2022 Quarter, ARLP delivered strong operating and financial performance with coal and oil & gas sales volumes, total revenues, net income and EBITDA all increasing significantly over the 2021 Quarter,” said Joseph W. Craft III, Chairman, President and Chief Executive Officer. “Our coal operations performed exceptionally well, particularly in light of the transportation challenges experienced during the 2022 Quarter, which resulted in delayed shipments of approximately 1.1 million tons. Through the efforts of our marketing teams, ARLP continued to benefit from rising coal markets as coal price realizations per ton increased $5.48 and $2.39 compared to the 2021 and Sequential Quarters, respectively. We also further strengthened our contract book during the 2022 Quarter, securing new agreements for the delivery of approximately 8.7 million tons through 2025 at prices well above our initial expectations. Higher energy prices and increased royalty volumes also drove strong performance by our royalties businesses, with both oil & gas royalties and coal royalties achieving a record EBITDA during the 2022 Quarter.”
Operating Results and Analysis
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
% Change |
|
|
|
|
|
|
|
|
|
|
2022 First |
|
2021 First |
|
Quarter / |
|
2021 Fourth |
|
% Change |
|||||
|
(in |
|
Quarter |
|
Quarter |
|
Quarter |
|
Quarter |
|
Sequential |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Coal Operations (1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Illinois Basin |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tons sold |
|
|
5.882 |
|
|
4.760 |
|
23.6 |
% |
|
|
6.329 |
|
(7.1) |
% |
|
Coal sales price per ton sold |
|
$ |
43.17 |
|
$ |
38.37 |
|
12.5 |
% |
|
$ |
41.63 |
|
3.7 |
% |
|
Segment Adjusted EBITDA Expense per ton |
|
$ |
30.19 |
|
$ |
26.38 |
|
14.4 |
% |
|
$ |
31.27 |
|
(3.5) |
% |
|
Segment Adjusted EBITDA |
|
$ |
78.2 |
|
$ |
57.7 |
|
35.6 |
% |
|
$ |
67.7 |
|
15.5 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Appalachia |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tons sold |
|
|
2.280 |
|
|
2.068 |
|
10.3 |
% |
|
|
2.771 |
|
(17.7) |
% |
|
Coal sales price per ton sold |
|
$ |
58.97 |
|
$ |
50.70 |
|
16.3 |
% |
|
$ |
53.30 |
|
10.6 |
% |
|
Segment Adjusted EBITDA Expense per ton |
|
$ |
36.72 |
|
$ |
35.65 |
|
3.0 |
% |
|
$ |
37.47 |
|
(2.0) |
% |
|
Segment Adjusted EBITDA |
|
$ |
51.1 |
|
$ |
31.5 |
|
62.2 |
% |
|
$ |
46.7 |
|
9.4 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Coal Operations |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tons sold |
|
|
8.162 |
|
|
6.828 |
|
19.5 |
% |
|
|
9.100 |
|
(10.3) |
% |
|
Coal sales price per ton sold |
|
$ |
47.58 |
|
$ |
42.10 |
|
13.0 |
% |
|
$ |
45.19 |
|
5.3 |
% |
|
Segment Adjusted EBITDA Expense per ton |
|
$ |
32.90 |
|
$ |
29.72 |
|
10.7 |
% |
|
$ |
33.86 |
|
(2.8) |
% |
|
Segment Adjusted EBITDA |
|
$ |
132.0 |
|
$ |
90.6 |
|
45.7 |
% |
|
$ |
116.4 |
|
13.4 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Royalties (1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Oil & Gas Royalties |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BOE sold (2) |
|
|
0.505 |
|
|
0.400 |
|
26.3 |
% |
|
|
0.458 |
|
10.3 |
% |
|
Oil percentage of BOE |
|
|
44.2 |
% |
|
48.4 |
% |
(8.7) |
% |
|
|
45.9 |
% |
(3.7) |
% |
|
Average sales price per BOE (3) |
|
$ |
61.26 |
|
$ |
35.02 |
|
74.9 |
% |
|
$ |
51.80 |
|
18.3 |
% |
|
Segment Adjusted EBITDA Expense |
|
$ |
3.0 |
|
$ |
2.1 |
|
45.8 |
% |
|
$ |
2.8 |
|
6.2 |
% |
|
Segment Adjusted EBITDA |
|
$ |
28.6 |
|
$ |
11.9 |
|
139.0 |
% |
|
$ |
22.4 |
|
27.6 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Coal Royalties |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Royalty tons sold |
|
|
5.553 |
|
|
4.521 |
|
22.8 |
% |
|
|
5.675 |
|
(2.1) |
% |
|
Revenue per royalty ton sold |
|
$ |
2.73 |
|
$ |
2.50 |
|
9.2 |
% |
|
$ |
2.64 |
|
3.4 |
% |
|
Segment Adjusted EBITDA Expense |
|
$ |
4.8 |
|
$ |
4.0 |
|
19.6 |
% |
|
$ |
5.1 |
|
(5.7) |
% |
|
Segment Adjusted EBITDA |
|
$ |
10.3 |
|
$ |
7.3 |
|
42.3 |
% |
|
$ |
9.9 |
|
4.0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Royalties |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total royalty revenues |
|
$ |
46.1 |
|
$ |
25.3 |
|
82.2 |
% |
|
$ |
39.4 |
|
16.9 |
% |
|
Segment Adjusted EBITDA Expense |
|
$ |
7.8 |
|
$ |
6.1 |
|
28.5 |
% |
|
$ |
7.9 |
|
(1.5) |
% |
|
Segment Adjusted EBITDA |
|
$ |
38.9 |
|
$ |
19.2 |
|
102.4 |
% |
|
$ |
32.3 |
|
20.4 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated Total (4) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues |
|
$ |
460.9 |
|
$ |
318.6 |
|
44.6 |
% |
|
$ |
473.5 |
|
(2.7) |
% |
|
Segment Adjusted EBITDA Expense |
|
$ |
261.2 |
|
$ |
197.7 |
|
32.1 |
% |
|
$ |
301.1 |
|
(13.3) |
% |
|
Segment Adjusted EBITDA |
|
$ |
170.9 |
|
$ |
109.8 |
|
55.6 |
% |
|
$ |
148.8 |
|
14.9 |
% |
_________________
|
(1) |
|
For definitions of Segment Adjusted EBITDA Expense and Segment Adjusted EBITDA and related reconciliations to comparable GAAP financial measures, please see the end of this release. Segment Adjusted EBITDA Expense per ton is defined as Segment Adjusted EBITDA Expense – Coal Operations (as reflected in the reconciliation table at the end of this release) divided by total tons sold. |
|
(2) |
|
Barrels of oil equivalent (“BOE”) for natural gas volumes is calculated on a 6:1 basis (6,000 cubic feet of natural gas to one barrel). |
|
(3) |
|
Average sales price per BOE is defined as oil & gas royalty revenues excluding lease bonus revenue divided by total BOE sold. |
|
(4) |
|
Reflects total consolidated results, which include our other and corporate activities and eliminations in addition to the Illinois Basin, Appalachia, Oil & Gas Royalties and Coal Royalties reportable segments highlighted above. |
ARLP’s coal sales prices per ton increased in all regions compared to both the 2021 and Sequential Quarters as a result of favorable market conditions. In the Illinois Basin, increased domestic prices and significantly higher export prices during the 2022 Quarter drove coal sales prices higher by 12.5% and 3.7% compared to the 2021 and Sequential Quarters, respectively. In Appalachia, sales prices increased by 16.3% and 10.6% compared to the 2021 and Sequential Quarters, respectively, primarily due to significantly higher export price realizations at our Mettiki and MC Mining operations. Coal sales volumes were higher by 23.6% in the Illinois Basin compared to the 2021 Quarter as a result of increased sales volumes across all mines in the region. In Appalachia, coal sales volumes increased 10.3% compared to the 2021 Quarter as a result of higher export volumes. Compared to the Sequential Quarter, shipment delays resulted in reduced coal sales volumes in both the Illinois Basin and Appalachian regions, which fell 7.1% and 17.7%, respectively, during the 2022 Quarter. ARLP ended the 2022 Quarter with total coal inventory of 1.6 million tons, representing a decrease of 0.2 million tons compared to the end of the 2021 Quarter and an increase of 1.0 million tons compared to the end of the Sequential Quarter.
Segment Adjusted EBITDA Expense per ton increased by 14.4% and 3.0% in the Illinois Basin and Appalachia, respectively, compared to the 2021 Quarter as a result of inflationary pressures on numerous expense items, including labor-related expenses and supply and maintenance costs, as well as longwall moves at our Hamilton, Tunnel Ridge and Mettiki mines. Lower recoveries at our Illinois Basin mines also contributed to increased per ton expenses in that region compared to the 2021 Quarter. Compared to the Sequential Quarter, Segment Adjusted EBITDA expense per ton decreased 3.5% and 2.0% in the Illinois Basin and Appalachia, respectively, primarily due to the previously discussed contract buy-out expense and unfavorable non-cash actuarial and accrual adjustments recognized in the Sequential Quarter.
For our Oil & Gas Royalties segment, significantly higher sales price realizations per BOE and increased volumes in the 2022 Quarter drove Segment Adjusted EBITDA higher by 139.0% to a record $28.6 million compared to $11.9 million for the 2021 Quarter. Compared to the Sequential Quarter, Segment Adjusted EBITDA increased by $6.2 million in the 2022 Quarter due to higher volumes, which increased 10.3%, and increased oil & gas prices, which rose by 18.3%.
Segment Adjusted EBITDA for our Coal Royalties segment increased 42.3% to a record $10.3 million for the 2022 Quarter compared to $7.3 million for the 2021 Quarter as a result of increased royalty tons sold and higher average royalty rates per ton.
Matrix and Alliance Technologies Group High
Level Strategy
Matrix Design Group, LLC (“Matrix”) is a wholly owned subsidiary of ARLP created in 2006 as a technical service group focused on deploying technology in domestic underground coal operations in response to the passage of The MINER Safety Act by the United States Congress. The Matrix strategy evolved over the last decade with a primary focus on supporting U.S. based coal mining operations with proximity detection equipment and other products and services. The customer base has grown to include international coal mining operations and expanded into non-coal mining applications. In 2021 Matrix had revenue of $22.7 million and EBITDA of $4.7 million. As factored into our 2022 consolidated guidance, we estimated revenue of approximately $35.0 million and EBITDA of $8.0 million for Matrix. Matrix is and always has been a cash flow positive business growing organically with limited capital investment to date.
We have more recently been developing Matrix as an incubator as a key component of our diversification strategy. Matrix and its subsidiaries today employ over 100 professionals covering hardware and software development, data analytics and AI technologies. There are many “AI companies” and many “software development” companies that exist but we believe Matrix has attributes which distinguish it from many of its peers. Matrix combines hardware, software and analytics in one platform, which is a powerful combination. Moreover, we believe the investments we are making into Francis Energy and Infinitum Electric (discussed below) have the potential to significantly benefit from the growing skills base, analytics and AI opportunities being developed by Matrix. These three companies are all focused in strong growth sectors aligned with the broader energy transition. Our long-term goals for these companies are to provide tangible offerings which are enhanced by this transition, rather than being dependent on it.
Over the past year, Matrix has embarked on a new strategy, which aims to position the company to compete in the energy transition space, while at the same time remaining committed to the markets they already serve. The reduction of base load coal powered generation resources from our country’s electrical grid combined with the anticipated growth of renewable power sources and the electric vehicle (“EV”) market are changing how electricity is generated, consumed and priced in the United States. As intermittent resources become a more significant portion of the energy mix, the market value of power will become more correlated with generation capacity in real time. This transition creates additional challenges for the electrical infrastructure in America, which was not designed to accommodate this load. ARLP’s relationships with electric utilities, industrial customers, and federal and state governments, along with our technology and manufacturing capabilities give us confidence that these challenges will provide growth opportunities for our Partnership.
Matrix is currently delivering products focused on data networking, communication and tracking systems, mining proximity detection systems, industrial collision avoidance systems, and data and analytics software. Future areas of potential investment by Matrix and ARLP’s broader management team focused on technology development (our “Alliance Technologies Group”) include smart cameras, energy storage, energy efficiency, renewable power generation, EV charging, smart metering and energy demand management.
Francis Energy
Earlier today Francis Renewable Energy, LLC (“Francis Energy”), an Oklahoma-based owner and operator of a leading comprehensive statewide network of EV fast charging infrastructure, with plans to service states across the Midwest and Eastern U.S., announced an equity investment by ARLP to help propel Francis Energy’s future growth. To date, Francis Energy has built a network with hundreds of fast chargers across Oklahoma and several other states, with a goal of providing EV drivers convenient, affordable, easy to use public access to charging stations.
The $1.2 trillion infrastructure bill that President Biden signed into law in November 2021 includes a large federal investment in electric vehicle charging infrastructure. The law authorizes $7.5 billion in federal spending available through two new programs to incentivize the buildout of EV infrastructure to eliminate range anxiety for EV drivers and support the booming growth of the U.S. electric vehicle market. The money will be allocated to private sector companies by federal, state and local governments creating opportunities for project developers and equipment manufacturers, like Matrix and Francis Energy.
Infinitum Electric Inc.
ARLP has also agreed to an equity investment in Infinitum Electric, Inc. (“Infinitum”), a Texas-based startup developer and manufacturer of electric motors featuring printed circuit board stators which have the potential to result in motors that are smaller, lighter, quieter, more efficient and capable of operating at a fraction of the carbon footprint of conventional electric motors. Infinitum’s products are supported by multiple patents and patent applications, which may have broad application across multiple industries.
Outlook
“Much has changed since we last released earnings in January,” said Mr. Craft. “We are excited to share our current energy transition strategy and the announcement of our investment in two entrepreneurial companies that we believe will provide significant returns for our unitholders within four to seven years. We are equally excited to continue supporting Matrix efforts to develop new product offerings that have the potential to grow their sales by 5 to 10 times over the same time period.”
Addressing ARLP’s current outlook, Mr. Craft added, “Commodity price realizations escalated dramatically during the 2022 Quarter, reflecting systemic supply shortages due, in part, to the impact of governmental policies over the last fifteen years and exacerbated by uncertainties created by Russia’s invasion of Ukraine in February of this year. Energy supplies have also been impacted by labor shortages along with supply chain and transportation disruptions. Demand has remained surprisingly strong despite Covid-19 related cases still impacting economies around the world, sanctions imposed on Russia and inflationary cost pressures in the United States not seen in 40 years.”
Mr. Craft added, “Forward pricing for worldwide commodities also rose dramatically during the 2022 Quarter, well above our initial expectations earlier this year. As a result, we updated 2022 full year guidance on April 26, 2022 (as shown below) as well as released our Board’s decision to increase ARLP’s cash distribution to unitholders by 40.0% to $0.35 for the 2022 Quarter. Future distributions will be considered by our Board on a quarterly basis. Consistent with our full-year guidance, management anticipates current market conditions continuing for the foreseeable future, which supports ARLP targeting increases to unitholder distributions of 10.0% to 15.0% per quarter over the balance of this year.”
ARLP’s updated guidance, as reported on April 26, 2022, is outlined below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2022 Full Year Guidance |
|||||
|
|
|
|
|
|
|
|
Coal Operations |
|
|
|
|
|
|
Volumes (Million Short Tons) |
|
|
|
|
|
|
Illinois Basin Sales Tons |
|
|
|
|
25.2 — 26.0 |
|
Appalachia Sales Tons |
|
|
|
|
10.3 — 11.0 |
|
Total Sales Tons |
|
|
|
|
35.5 — 37.0 |
|
|
|
|
|
|
|
|
Committed |
|
|
|
|
|
|
2022 — Domestic/Export/Total |
|
|
|
|
30.1/4.1/34.2 |
|
2023 — Domestic/Export/Total |
|
|
|
|
17.9/2.0/19.9 |
|
|
|
|
|
|
|
|
Per |
|
|
|
|
|
|
Coal Sales Price per ton sold (1) |
|
|
|
|
$54.00 — $63.00 |
|
Segment Adjusted EBITDA Expense per ton sold (2) |
|
|
|
|
$33.50 — $35.50 |
|
|
|
|
|
|
|
|
Royalties |
|
|
|
|
|
|
Oil & Gas Royalties |
|
|
|
|
|
|
Oil (000 Barrels) |
|
|
|
|
885 – 935 |
|
Natural gas (000 MCF) |
|
|
|
|
3,000 – 3,400 |
|
Liquids (000 Barrels) |
|
|
|
|
350 – 380 |
|
Segment Adjusted EBITDA Expense (% of Oil & Gas Royalties Revenue) |
|
|
|
|
~ 12.0% |
|
|
|
|
|
|
|
|
Coal Royalties |
|
|
|
|
|
|
Royalty tons sold (Million Short Tons) |
|
|
|
|
21.5 — 22.0 |
|
Revenue per royalty ton sold |
|
|
|
|
$3.10 — $3.20 |
|
Segment Adjusted EBITDA Expense per royalty ton sold |
|
|
|
|
$1.10 — $1.20 |
|
|
|
|
|
|
|
|
Consolidated (Millions) |
|
|
|
|
|
|
Depreciation, depletion and amortization |
|
|
|
|
$260 — $270 |
|
General and administrative |
|
|
|
|
$82 — $84 |
|
Net interest expense |
|
|
|
|
$37 — $38 |
|
Income tax expense |
|
|
|
|
$53 — $55 |
|
Capital expenditures |
|
|
|
|
$220 — $240 |
_________________
|
(1) |
Sales price per ton is defined as total coal sales revenue divided by total tons sold. |
|
|
(2) |
Segment Adjusted EBITDA Expense is defined as operating expenses, coal purchases and other expense. |
A conference call regarding ARLP’s 2021 Quarter financial results is scheduled for today at 10:00 a.m. Eastern. To participate in the conference call, dial (877) 407-0784 and request to be connected to the Alliance Resource Partners, L.P. earnings conference call. International callers should dial (201) 689-8560 and request to be connected to the same call. Investors may also listen to the call via the “investor information” section of ARLP’s website at http://www.arlp.com.
An audio replay of the conference call will be available for approximately one week. To access the audio replay, dial U.S. Toll Free (844) 512-2921; International Toll (412) 317-6671 and request to be connected to replay using access code 13729024.
About Alliance Resource Partners, L.P.
ARLP is a diversified energy company that is currently the second largest coal producer in the United States. ARLP also generates operating and royalty income from mineral interests it owns in strategic coal and oil & gas producing regions in the United States. In addition, ARLP is positioning itself as an energy provider for the future by leveraging its core technology and operating competencies to make strategic investments in the fast-growing energy and infrastructure transition.
News, unit prices and additional information about ARLP, including filings with the Securities and Exchange Commission (“SEC”), are available at http://www.arlp.com. For more information, contact the investor relations department of ARLP at (918) 295-7674 or via e-mail at investorrelations@arlp.com.
About Matrix Design Group, LLC
Matrix is an ISO 9001 certified designer, developer and marketer of safety and productivity technology for use in mining and industrial applications. Its innovative, industry-leading systems include products focused on data networking, communication and tracking systems, mining proximity detection systems, industrial collision avoidance systems, and data and analytics software. Headquartered in Newburgh, Indiana, Matrix has offices in Lexington, KY, Johannesburg and Pretoria, South Africa and service locations throughout its mining regions.
The statements and projections used throughout this release are based on current expectations. These statements and projections are forward-looking, and actual results may differ materially. These projections do not include the potential impact of any mergers, acquisitions or other business combinations that may occur after the date of this release. We have included more information below regarding business risks that could affect our results.
FORWARD-LOOKING STATEMENTS: With the exception
of historical matters, any matters discussed in this press release are
forward-looking statements that involve risks and uncertainties that could
cause actual results to differ materially from projected results. Those
forward-looking statements include expectations with respect to coal and oil
& gas consumption and expected future prices, our ability to increase
unitholder distributions in future quarters, business plans and potential growth
with respect to Matrix, Francis Energy and Infinitum, optimizing cash flows,
reducing operating and capital expenditures, preserving liquidity and
maintaining financial flexibility, among others. These risks to our ability to
achieve these outcomes include, but are not limited to, the following: the
outcome or escalation of current hostilities in Ukraine, the severity,
magnitude, and duration of the COVID-19 pandemic and the emergence of new virus
variants, including impacts of the pandemic and of businesses’ and governments’
responses to the pandemic, including actions to mitigate its impact and the
development of treatments and vaccines, on our operations and personnel, and on
demand for coal, oil, and natural gas, the financial condition of our customers
and suppliers, available liquidity and capital sources and broader economic
disruptions; changes in macroeconomic and market conditions and market
volatility arising from hostilities in Ukraine, the COVID-19 pandemic or
otherwise, including inflation, changes in coal, oil, natural gas, and natural
gas liquids prices, and the impact of such changes and volatility on our
financial position; decline in the coal industry’s share of electricity
generation, including as a result of environmental concerns related to coal
mining and combustion and the cost and perceived benefits of other sources of
electricity and fuels, such as oil & gas, nuclear energy, and renewable
fuels; changes in global economic and geo-political conditions or in industries
in which our customers operate; changes in coal prices and/or oil & gas
prices, demand and availability which could affect our operating results and
cash flows; actions of the major oil-producing countries with respect to oil
production volumes and prices could have direct and indirect impacts over the
near and long term on oil & gas exploration and production operations at
the properties in which we hold mineral interests; changes in competition in
domestic and international coal markets and our ability to respond to such
changes; potential shut-ins of production by operators of the properties in
which we hold mineral interests due to low oil, natural gas, and natural gas
liquid prices or the lack of downstream demand or storage capacity; risks
associated with the expansion of our operations and properties; our ability to
identify and complete acquisitions; the success of our development plans for
Matrix and our investments in Francis Energy and Infinitum which are emerging
infrastructure and technology companies; dependence on significant customer
contracts, including renewing existing contracts upon expiration; adjustments
made in price, volume, or terms to existing coal supply agreements; the effects
of and changes in trade, monetary and fiscal policies and laws, including the
interest rate policies of the Federal Reserve Board; the effects of and changes
in taxes or tariffs and other trade measures adopted by the United States and
foreign governments; legislation, regulations, and court decisions and
interpretations thereof, both domestic and foreign, including those relating to
the environment and the release of greenhouse gases, mining, miner health and
safety, hydraulic fracturing, and health care; deregulation of the electric
utility industry or the effects of any adverse change in the coal industry,
electric utility industry, or general economic conditions; investors’ and other
stakeholders’ increasing attention to environmental, social, and governance
matters; liquidity constraints, including those resulting from any future
unavailability of financing; customer bankruptcies, cancellations or breaches
to existing contracts, or other failures to perform; customer delays, failure
to take coal under contracts or defaults in making payments; our productivity
levels and margins earned on our coal sales; disruptions to oil & gas
exploration and production operations at the properties in which we hold
mineral interests; changes in raw material costs, including due to inflationary
pressures; changes in our ability to recruit, hire and maintain labor,
including, as a result of, the potential impact of government-imposed vaccine
mandates; our ability to maintain satisfactory relations with our employees;
increases in labor costs including costs of health insurance and taxes resulting
from the Affordable Care Act, adverse changes in work rules, or cash payments
or projections associated with workers’ compensation claims; increases in
transportation costs and risk of transportation delays or interruptions;
operational interruptions due to geologic, permitting, labor, weather, supply
chain shortages of equipment or mine supplies, or other factors; risks
associated with major mine-related accidents, mine fires, mine floods or other
interruptions; results of litigation, including claims not yet asserted;
foreign currency fluctuations that could adversely affect the competitiveness
of our coal abroad; difficulty maintaining our surety bonds for mine
reclamation as well as workers’ compensation and black lung benefits;
difficulty in making accurate assumptions and projections regarding post-mine
reclamation as well as pension, black lung benefits, and other post-retirement
benefit liabilities; uncertainties in estimating and replacing our coal mineral
reserves and resources; uncertainties in estimating and replacing our oil &
gas reserves; uncertainties in the amount of oil & gas production due to
the level of drilling and completion activity by the operators of our oil &
gas properties; uncertainties in the future of the electric vehicle industry
and the market for EV charging stations; the impact of current and potential
changes to federal or state tax rules and regulations, including a loss or
reduction of benefits from certain tax deductions and credits; difficulty
obtaining commercial property insurance, and risks associated with our
participation in the commercial insurance property program; evolving
cybersecurity risks, such as those involving unauthorized access,
denial-of-service attacks, malicious software, data privacy breaches by employees,
insiders or others with authorized access, cyber or phishing-attacks,
ransomware, malware, social engineering, physical breaches, or other actions;
and difficulty in making accurate assumptions and projections regarding future
revenues and costs associated with equity investments in companies we do not
control.
Additional information concerning these and
other factors can be found in ARLP’s public periodic filings with the SEC,
including ARLP’s Annual Report on Form 10-K for the year ended December 31,
2021, filed on February 25, 2022 with the SEC. Except as required by applicable
securities laws, ARLP does not intend to update its forward-looking statements.
|
ALLIANCE RESOURCE PARTNERS, L.P. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF INCOME AND OPERATING (In thousands, except unit and per unit data) (Unaudited) |
|||||||||
|
|
|
|
|
|
|
|
|
||
|
|
|
Three Months Ended |
|
||||||
|
|
|
March 31, |
|
||||||
|
|
|
2022 |
|
2021 |
|
||||
|
|
|
|
|
|
|
|
|
||
|
Tons Sold |
|
|
8,162 |
|
|
|
6,828 |
|
|
|
Tons |
|
|
9,178 |
|
|
|
8,001 |
|
|
|
Mineral Interest Volumes (BOE) |
|
|
505 |
|
|
|
400 |
|
|
|
|
|
|
|
|
|
|
|
||
|
SALES AND OPERATING REVENUES: |
|
|
|
|
|
|
|
||
|
Coal sales |
|
$ |
388,360 |
|
|
$ |
287,487 |
|
|
|
Oil & gas royalties |
|
|
30,927 |
|
|
|
13,999 |
|
|
|
Transportation revenues |
|
|
29,372 |
|
|
|
11,068 |
|
|
|
Other revenues |
|
|
12,204 |
|
|
|
6,068 |
|
|
|
Total revenues |
|
|
460,863 |
|
|
|
318,622 |
|
|
|
|
|
|
|
|
|
|
|
||
|
EXPENSES: |
|
|
|
|
|
|
|
||
|
Operating expenses (excluding depreciation, depletion and amortization) |
|
|
261,746 |
|
|
|
196,520 |
|
|
|
Transportation expenses |
|
|
29,372 |
|
|
|
11,068 |
|
|
|
General and administrative |
|
|
18,596 |
|
|
|
15,504 |
|
|
|
Depreciation, depletion and amortization |
|
|
63,314 |
|
|
|
59,202 |
|
|
|
Total operating expenses |
|
|
373,028 |
|
|
|
282,294 |
|
|
|
|
|
|
|
|
|
|
|
||
|
INCOME FROM OPERATIONS |
|
|
87,835 |
|
|
|
36,328 |
|
|
|
|
|
|
|
|
|
|
|
||
|
Interest expense, net |
|
|
(9,662 |
) |
|
|
(10,396 |
) |
|
|
Interest income |
|
|
35 |
|
|
|
17 |
|
|
|
Equity method investment income |
|
|
883 |
|
|
|
62 |
|
|
|
Other income (expense) |
|
|
566 |
|
|
|
(1,197 |
) |
|
|
INCOME BEFORE INCOME TAXES |
|
|
79,657 |
|
|
|
24,814 |
|
|
|
|
|
|
|
|
|
|
|
||
|
INCOME TAX EXPENSE (BENEFIT) |
|
|
42,715 |
|
|
|
(12 |
) |
|
|
|
|
|
|
|
|
|
|
||
|
NET INCOME |
|
|
36,942 |
|
|
|
24,826 |
|
|
|
|
|
|
|
|
|
|
|
||
|
LESS: NET INCOME ATTRIBUTABLE TO NONCONTROLLING INTEREST |
|
|
(290 |
) |
|
|
(78 |
) |
|
|
|
|
|
|
|
|
|
|
||
|
NET INCOME ATTRIBUTABLE TO ARLP |
|
$ |
36,652 |
|
|
$ |
24,748 |
|
|
|
|
|
|
|
|
|
|
|
||
|
EARNINGS PER LIMITED PARTNER UNIT – |
|
$ |
0.28 |
|
|
$ |
0.19 |
|
|
|
|
|
|
|
|
|
|
|
||
|
WEIGHTED-AVERAGE NUMBER OF UNITS |
|
|
127,195,219 |
|
|
|
127,195,219 |
|
|
|
ALLIANCE RESOURCE PARTNERS, L.P. AND SUBSIDIARIES
CONDENSED CONSOLIDATED BALANCE SHEETS (In thousands, except unit data) (Unaudited) |
|||||||||
|
|
|
|
|
|
|
|
|
||
|
|
|
March 31, |
|
December 31, |
|
||||
|
|
|
2022 |
|
2021 |
|
||||
|
ASSETS |
|
|
|
|
|
|
|
||
|
CURRENT |
|
|
|
|
|
|
|
||
|
Cash and cash equivalents |
|
$ |
128,191 |
|
|
$ |
122,403 |
|
|
|
Trade receivables |
|
|
156,698 |
|
|
|
129,531 |
|
|
|
Other receivables |
|
|
440 |
|
|
|
680 |
|
|
|
Inventories, net |
|
|
95,745 |
|
|
|
60,302 |
|
|
|
Advance royalties |
|
|
4,385 |
|
|
|
4,958 |
|
|
|
Prepaid expenses and other assets |
|
|
19,238 |
|
|
|
21,354 |
|
|
|
Total current assets |
|
|
404,697 |
|
|
|
339,228 |
|
|
|
PROPERTY, |
|
|
|
|
|
|
|
||
|
Property, plant and equipment, at cost |
|
|
3,666,987 |
|
|
|
3,608,347 |
|
|
|
Less accumulated depreciation, depletion and amortization |
|
|
(1,975,381 |
) |
|
|
(1,909,669 |
) |
|
|
Total property, plant and equipment, net |
|
|
1,691,606 |
|
|
|
1,698,678 |
|
|
|
OTHER |
|
|
|
|
|
|
|
||
|
Advance royalties |
|
|
71,403 |
|
|
|
63,524 |
|
|
|
Equity method investments |
|
|
26,194 |
|
|
|
26,325 |
|
|
|
Goodwill |
|
|
4,373 |
|
|
|
4,373 |
|
|
|
Operating lease right-of-use assets |
|
|
15,165 |
|
|
|
14,158 |
|
|
|
Other long-term assets |
|
|
12,109 |
|
|
|
13,120 |
|
|
|
Total other assets |
|
|
129,244 |
|
|
|
121,500 |
|
|
|
TOTAL ASSETS |
|
$ |
2,225,547 |
|
|
$ |
2,159,406 |
|
|
|
|
|
|
|
|
|
|
|
||
|
LIABILITIES AND PARTNERS’ CAPITAL |
|
|
|
|
|
|
|
||
|
CURRENT |
|
|
|
|
|
|
|
||
|
Accounts payable |
|
$ |
92,904 |
|
|
$ |
69,586 |
|
|
|
Accrued taxes other than income taxes |
|
|
15,209 |
|
|
|
17,787 |
|
|
|
Accrued payroll and related expenses |
|
|
31,178 |
|
|
|
36,805 |
|
|
|
Accrued interest |
|
|
12,500 |
|
|
|
5,000 |
|
|
|
Workers’ compensation and pneumoconiosis benefits |
|
|
12,293 |
|
|
|
12,293 |
|
|
|
Current finance lease obligations |
|
|
665 |
|
|
|
840 |
|
|
|
Current operating lease obligations |
|
|
2,133 |
|
|
|
1,820 |
|
|
|
Other current liabilities |
|
|
19,815 |
|
|
|
17,375 |
|
|
|
Current maturities, long-term debt, net |
|
|
15,359 |
|
|
|
16,071 |
|
|
|
Total current liabilities |
|
|
202,056 |
|
|
|
177,577 |
|
|
|
LONG-TERM LIABILITIES: |
|
|
|
|
|
|
|
||
|
Long-term debt, excluding current maturities, net |
|
|
415,990 |
|
|
|
418,942 |
|
|
|
Pneumoconiosis benefits |
|
|
108,491 |
|
|
|
107,560 |
|
|
|
Accrued pension benefit |
|
|
24,857 |
|
|
|
25,590 |
|
|
|
Workers’ compensation |
|
|
44,669 |
|
|
|
44,911 |
|
|
|
Asset retirement obligations |
|
|
123,989 |
|
|
|
123,517 |
|
|
|
Long-term finance lease obligations |
|
|
590 |
|
|
|
618 |
|
|
|
Long-term operating lease obligations |
|
|
13,009 |
|
|
|
12,366 |
|
|
|
Deferred income tax liabilities |
|
|
37,621 |
|
|
|
391 |
|
|
|
Other liabilities |
|
|
20,882 |
|
|
|
21,865 |
|
|
|
Total long-term liabilities |
|
|
790,098 |
|
|
|
755,760 |
|
|
|
Total liabilities |
|
|
992,154 |
|
|
|
933,337 |
|
|
|
|
|
|
|
|
|
|
|
||
|
COMMITMENTS |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
|
PARTNERS’ |
|
|
|
|
|
|
|
||
|
ARLP Partners’ Capital: |
|
|
|
|
|
|
|
||
|
Limited Partners – Common Unitholders 127,195,219 units outstanding |
|
|
1,285,725 |
|
|
|
1,279,183 |
|
|
|
Accumulated other comprehensive loss |
|
|
(63,439 |
) |
|
|
(64,229 |
) |
|
|
Total ARLP Partners’ Capital |
|
|
1,222,286 |
|
|
|
1,214,954 |
|
|
|
Noncontrolling interest |
|
|
11,107 |
|
|
|
11,115 |
|
|
|
Total Partners’ Capital |
|
|
1,233,393 |
|
|
|
1,226,069 |
|
|
|
TOTAL LIABILITIES AND PARTNERS’ CAPITAL |
|
$ |
2,225,547 |
|
|
$ |
2,159,406 |
|
|
|
ALLIANCE RESOURCE PARTNERS, L.P. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS (In thousands) (Unaudited) |
|||||||||
|
|
|
|
|
|
|
|
|
||
|
|
|
Three Months Ended |
|
||||||
|
|
|
March 31, |
|
||||||
|
|
|
2022 |
|
2021 |
|
||||
|
|
|
|
|
|
|
|
|
||
|
CASH FLOWS FROM OPERATING ACTIVITIES |
|
$ |
89,036 |
|
|
$ |
54,647 |
|
|
|
|
|
|
|
|
|
|
|
||
|
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
||
|
Property, plant and equipment: |
|
|
|
|
|
|
|
||
|
Capital expenditures |
|
|
(59,153 |
) |
|
|
(31,437 |
) |
|
|
Increase in accounts payable and accrued liabilities |
|
|
13,551 |
|
|
|
7,200 |
|
|
|
Proceeds from sale of property, plant and equipment |
|
|
928 |
|
|
|
1,139 |
|
|
|
Distributions received from investments in excess of cumulative earnings |
|
|
131 |
|
|
|
361 |
|
|
|
Other |
|
|
(982 |
) |
|
|
— |
|
|
|
Net cash used in investing activities |
|
|
(45,525 |
) |
|
|
(22,737 |
) |
|
|
|
|
|
|
|
|
|
|
||
|
CASH |
|
|
|
|
|
|
|
||
|
Payments under securitization facility |
|
|
— |
|
|
|
(17,800 |
) |
|
|
Payments on equipment financings |
|
|
(4,472 |
) |
|
|
(4,239 |
) |
|
|
Borrowings under revolving credit facilities |
|
|
— |
|
|
|
10,000 |
|
|
|
Payments under revolving credit facilities |
|
|
— |
|
|
|
(42,500 |
) |
|
|
Borrowings from line of credit |
|
|
— |
|
|
|
1,830 |
|
|
|
Payments on finance lease obligations |
|
|
(203 |
) |
|
|
(185 |
) |
|
|
Payment of debt issuance costs |
|
|
— |
|
|
|
(6 |
) |
|
|
Distributions paid to Partners |
|
|
(32,750 |
) |
|
|
— |
|
|
|
Other |
|
|
(298 |
) |
|
|
(141 |
) |
|
|
Net cash used in financing activities |
|
|
(37,723 |
) |
|
|
(53,041 |
) |
|
|
|
|
|
|
|
|
|
|
||
|
NET |
|
|
5,788 |
|
|
|
(21,131 |
) |
|
|
|
|
|
|
|
|
|
|
||
|
CASH |
|
|
122,403 |
|
|
|
55,574 |
|
|
|
|
|
|
|
|
|
|
|
||
|
CASH |
|
$ |
128,191 |
|
|
$ |
34,443 |
|
|
Reconciliation of
GAAP “net income attributable to ARLP” to non-GAAP “EBITDA”
and “Distributable Cash Flow” (in thousands).
EBITDA is defined as net income attributable to ARLP before net interest expense, income taxes and depreciation, depletion and amortization. Distributable cash flow (“DCF”) is defined as EBITDA excluding interest expense (before capitalized interest), interest income, income taxes and estimated maintenance capital expenditures. Distribution coverage ratio (“DCR”) is defined as DCF divided by distributions paid to partners.
Management believes that the presentation of such additional financial measures provides useful information to investors regarding our performance and results of operations because these measures, when used in conjunction with related GAAP financial measures, (i) provide additional information about our core operating performance and ability to generate and distribute cash flow, (ii) provide investors with the financial analytical framework upon which management bases financial, operational, compensation and planning decisions and (iii) present measurements that investors, rating agencies and debt holders have indicated are useful in assessing us and our results of operations.
EBITDA, DCF and DCR should not be considered as alternatives to net income attributable to ARLP, net income, income from operations, cash flows from operating activities or any other measure of financial performance presented in accordance with GAAP. EBITDA and DCF are not intended to represent cash flow and do not represent the measure of cash available for distribution. Our method of computing EBITDA, DCF and DCR may not be the same method used to compute similar measures reported by other companies, or EBITDA, DCF and DCR may be computed differently by us in different contexts (i.e. public reporting versus computation under financing agreements).
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
Three Months Ended |
|
Three Months Ended |
|
||||||||
|
|
|
March 31, |
|
December 31, |
|
||||||||
|
|
|
2022 |
|
2021 |
|
2021 |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
Net income attributable to ARLP |
|
$ |
36,652 |
|
|
$ |
24,748 |
|
|
$ |
51,826 |
|
|
|
Depreciation, depletion and amortization |
|
|
63,314 |
|
|
|
59,202 |
|
|
|
68,679 |
|
|
|
Interest expense, net |
|
|
9,697 |
|
|
|
10,465 |
|
|
|
9,628 |
|
|
|
Capitalized interest |
|
|
(70 |
) |
|
|
(86 |
) |
|
|
(82 |
) |
|
|
Income tax expense (benefit) |
|
|
42,715 |
|
|
|
(12 |
) |
|
|
190 |
|
|
|
EBITDA |
|
|
152,308 |
|
|
|
94,317 |
|
|
|
130,241 |
|
|
|
Interest expense, net |
|
|
(9,697 |
) |
|
|
(10,465 |
) |
|
|
(9,628 |
) |
|
|
Income tax (expense) benefit |
|
|
(42,715 |
) |
|
|
12 |
|
|
|
(190 |
) |
|
|
Deferred income tax expense (benefit) (1) |
|
|
37,294 |
|
|
|
(12 |
) |
|
|
123 |
|
|
|
Estimated maintenance capital expenditures (2) |
|
|
(51,947 |
) |
|
|
(39,205 |
) |
|
|
(42,821 |
) |
|
|
Distributable Cash Flow |
|
$ |
85,243 |
|
|
$ |
44,647 |
|
|
$ |
77,725 |
|
|
|
Distributions paid to partners |
|
$ |
32,750 |
|
|
$ |
— |
|
|
$ |
26,072 |
|
|
|
Distribution Coverage Ratio |
|
|
2.60 |
|
|
|
— |
|
|
|
2.98 |
|
|
_________________
|
(1) |
Deferred income tax expense (benefit) is the amount of income tax expense (benefit) during the period on temporary differences between the tax basis and financial reporting basis of recorded assets and liabilities. These differences generally arise in one period and reverse in subsequent periods to eventually offset each other and do not impact the amount of distributable cash flow available to be paid to partners. |
|
|
(2) |
Maintenance capital expenditures are those capital expenditures required to maintain, over the long-term, the existing infrastructure of our coal assets. We estimate maintenance capital expenditures on an annual basis based upon a five-year planning horizon. For the 2022 planning horizon, average annual estimated maintenance capital expenditures are assumed to be $5.66 per ton produced compared to an estimated $4.90 per ton produced in 2021. Our actual maintenance capital expenditures fluctuate depending on various factors, including maintenance schedules and timing of capital projects, among others. |
Reconciliation of
GAAP “Cash flows from operating activities” to non-GAAP “Free
cash flow” (in thousands).
Free cash flow is defined as cash flows from operating activities less capital expenditures. Free cash flow should not be considered as an alternative to cash flows from operating activities or any other measure of financial performance presented in accordance with GAAP. Our method of computing free cash flow may not be the same method used by other companies. Free cash flow is a supplemental liquidity measure used by our management to assess our ability to generate excess cash flow from our operations.
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
Three Months Ended |
|
Three Months Ended |
|
||||||||
|
|
|
March 31, |
|
December 31, |
|
||||||||
|
|
|
2022 |
|
2021 |
|
2021 |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
Cash flows from operating activities |
|
$ |
89,036 |
|
|
$ |
54,647 |
|
|
$ |
114,225 |
|
|
|
Capital expenditures |
|
|
(59,153 |
) |
|
|
(31,437 |
) |
|
|
(34,323 |
) |
|
|
Free cash flow |
|
$ |
29,883 |
|
|
$ |
23,210 |
|
|
$ |
79,902 |
|
|
Reconciliation of
GAAP “Operating Expenses” to non-GAAP “Segment Adjusted EBITDA
Expense” and Reconciliation of non-GAAP ” EBITDA” to
“Segment Adjusted EBITDA” (in thousands).
Segment Adjusted EBITDA Expense includes operating expenses, coal purchases and other expense. Transportation expenses are excluded as these expenses are passed through to our customers and, consequently, we do not realize any margin on transportation revenues. Segment Adjusted EBITDA Expense is used as a supplemental financial measure by our management to assess the operating performance of our segments. Segment Adjusted EBITDA Expense is a key component of EBITDA in addition to coal sales, royalty revenues and other revenues. The exclusion of corporate general and administrative expenses from Segment Adjusted EBITDA Expense allows management to focus solely on the evaluation of segment operating performance as it primarily relates to our operating expenses. Segment Adjusted EBITDA Expense – Coal Operations excludes expenses of our Oil & Gas Royalties segment and is adjusted for intercompany interactions with our Coal Royalties segment.
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
Three Months Ended |
|
Three Months Ended |
|
||||||||
|
|
|
March 31, |
|
December 31, |
|
||||||||
|
|
|
2022 |
|
2021 |
|
2021 |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
Operating expense |
|
$ |
261,746 |
|
|
$ |
196,520 |
|
|
$ |
300,497 |
|
|
|
Outside coal purchases |
|
|
— |
|
|
|
— |
|
|
|
193 |
|
|
|
Other expense (income) |
|
|
(566 |
) |
|
|
1,197 |
|
|
|
388 |
|
|
|
Segment Adjusted EBITDA Expense |
|
|
261,180 |
|
|
|
197,717 |
|
|
|
301,078 |
|
|
|
Segment Adjusted EBITDA Expense – Oil & Gas Royalties |
|
|
(3,001 |
) |
|
|
(2,058 |
) |
|
|
(2,827 |
) |
|
|
Segment Adjusted EBITDA Expense – Coal Royalties |
|
|
(4,819 |
) |
|
|
(4,028 |
) |
|
|
(5,112 |
) |
|
|
Intercompany coal royalties (1) |
|
|
15,167 |
|
|
|
11,301 |
|
|
|
14,992 |
|
|
|
Segment Adjusted EBITDA Expense – Coal Operations |
|
$ |
268,527 |
|
|
$ |
202,932 |
|
|
$ |
308,131 |
|
|
_________________
|
(1) |
Intercompany coal royalties earned by our Coal Royalties segment represent coal royalty expense incurred by our operating mines and are therefore added back to consolidated Segment Adjusted EBITDA Expense to reflect Segment Adjusted EBITDA Expense – Coal Operations. |
Segment Adjusted EBITDA is defined as net income attributable to ARLP before net interest expense, income taxes, depreciation, depletion and amortization and general and administrative expenses. Segment Adjusted EBITDA – Coal Operations excludes the contribution of our Oil & Gas and Coal Royalties segments to allow management to focus solely on the operating performance of our Illinois Basin and Appalachia segments.
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
Three Months Ended |
|
Three Months Ended |
|
||||||||
|
|
|
March 31, |
|
December 31, |
|
||||||||
|
|
|
2022 |
|
2021 |
|
2021 |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
EBITDA (See reconciliation to GAAP above) |
|
$ |
152,308 |
|
|
$ |
94,317 |
|
|
$ |
130,241 |
|
|
|
General and administrative |
|
|
18,596 |
|
|
|
15,504 |
|
|
|
18,509 |
|
|
|
Segment Adjusted EBITDA |
|
|
170,904 |
|
|
|
109,821 |
|
|
|
148,750 |
|
|
|
Segment Adjusted EBITDA – Total Royalties |
|
|
(38,900 |
) |
|
|
(19,219 |
) |
|
|
(32,318 |
) |
|
|
Segment Adjusted EBITDA – Coal Operations |
|
$ |
132,004 |
|
|
$ |
90,602 |
|
|
$ |
116,432 |
|
|
Brian L. Cantrell
Alliance Resource Partners, L.P.
(918) 295-7673
Source: Alliance Resource Partners, L.P.
Release – Element79 Gold Appoints Antonios Maragakis to Board of Directors
Element79 Gold Appoints Antonios Maragakis to Board of Directors
News and Market Data on Element79 Gold
VANCOUVER, BC /
ACCESSWIRE / May 2, 2022 / Element79 Gold Corp. (CSE:ELEM)(OTC PINK:ELMGF)(FSE:7YS) (“Element79 Gold”, the “Company“) is pleased to announce the appointment of Antonios Maragakis as a director of the Company. Mr. Maragakis will also continue to serve as Chief Operating Officer of the Company.
Mr. Maragakis will be increasing his management and advisement capacity for the Company in addition to his current role as Element79 Gold’s COO. Mr. Maragakis holds a distinguished resume, including management and director-level positions overseeing multi-billion dollar project portfolios internationally at organizations including:
- Barrick
Gold Corporation, where he led projects in North America across a portfolio of 70+ projects worth over $2.3 billion - Skeena
Resources Limited, where he worked closely with executive leadership to develop the Eskay Creek Project - Freeport-McMoRan
Inc., where he helped develop the $3 billion Indonesian Copper Smelter Project - Eldorado
Gold Corporation, where he was Project Director for the over $1 billion Skouries Project as well as leading the operational turnaround of the Kassandra Mines - Koch
Industries (the largest privately held company in the U.S.1), where he helped execute the Enid Expansion Megaproject
When asked about Mr. Maragakis’ appointment, Element79 Gold CEO James Tworek said, “Tony’s increased management and leadership in Element 79 will help us successfully set up the company to proceed quickly from an exploration company to a producer”.
Mr. Maragakis is currently residing in Peru with his family to oversee the Company’s acquisition of Calipuy Resources Inc. (information regarding this news release dated March 10th, 2022, is available here). “We are creating a culture of success, establishing the teams and systems that support a dynamic mining company that can explore, engineer and produce mines efficiently and effectively”.
About Element79
Gold
Element79 Gold is a mineral exploration company focused on the acquisition, exploration and development of mining properties for gold and associated metals. Element79 Gold has acquired its flagship Maverick Springs Project located in the famous gold mining district of northeastern Nevada, USA, between the Elko and White Pine Counties, where it has recently completed a 43-101-compliant, pit-constrained mineral resource estimate reflecting an Inferred resource of 3.71 million ounces of gold equivalent* “AuEq” at a grade of 0.92 g/t AuEq (0.34 g/t Au and 43.4 g/t Ag)) with an effective date of Feb. 4, 2022. The acquisition of the Maverick Springs Project also included a portfolio of 15 properties along the Battle Mountain trend in Nevada, which the Company is analyzing for further merit of exploration, along with the potential for sale or spin-out. In British Columbia, Element79 Gold has executed a Letter of Intent to acquire a private company which holds the option to 100% interest of the Snowbird High-Grade Gold Project, which consists of 10 mineral claims located in Central British Columbia, approximately 20km west of Fort St. James. In Peru, Element79 Gold has signed a letter of intent to acquire the business and assets of Calipuy Resources Inc., which holds 100% interest in the past-producing Lucero Mine, one of the highest-grade underground mines to be commercially mined in Peru’s history, as well as the past-producing Machacala Mine. The Company also has an option to acquire 100% interest in the Dale Property which consists of 90 unpatented mining claims located approximately 100 km southwest of Timmins, Ontario, Canada in the Timmins Mining Division, Dale Township. For more information about the Company, please visit www.element79.gold or www.element79gold.com.
On Behalf of the Company
James Tworek
CEO
Contact Information
Investor Relations Department
Phone: +1 (604) 200-3608
E-mail: investors@element79.gold
Cautionary Note
Regarding Forward Looking Statements
This press contains “forward?looking information” and “forward-looking statements” under applicable securities laws (collectively, “forward?looking statements”). These statements relate to future events or the Company’s future performance, business prospects or opportunities that are based on forecasts of future results, estimates of amounts not yet determinable and assumptions of management made in light of management’s experience and perception of historical trends, current conditions and expected future developments. Forward-looking statements include, but are not limited to, statements with respect to: the Company’s business strategy; future planning processes; exploration activities; the timing and result of exploration activities; capital projects and exploration activities and the possible results thereof; acquisition opportunities; and the impact of acquisitions, if any, on the Company. Assumptions may prove to be incorrect and actual results may differ materially from those anticipated. Consequently, forward-looking statements cannot be guaranteed. As such, investors are cautioned not to place undue reliance upon forward-looking statements as there can be no assurance that the plans, assumptions or expectations upon which they are placed will occur. All statements other than statements of historical fact may be forward?looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives or future events or performance (often, but not always, using words or phrases such as “seek”, “anticipate”, “plan”, “continue”, “estimate”, “expect”, “may”, “will”, “project”, “predict”, “forecast”, “potential”, “target”, “intend”, “could”, “might”, “should”, “believe” and similar expressions) are not statements of historical fact and may be “forward?looking statements”.
Actual results may vary from forward-looking statements. Forward-looking statements are subject to known and unknown risks, uncertainties and other factors that may cause actual results to materially differ from those expressed or implied by such forward-looking statements, including but not limited to: the duration and effects of the coronavirus and COVID-19; risks related to the integration of acquisitions; actual results of exploration activities; conclusions of economic evaluations; changes in project parameters as plans continue to be refined; commodity prices; variations in ore reserves, grade or recovery rates; actual performance of plant, equipment or processes relative to specifications and expectations; accidents; labour relations; relations with local communities; changes in national or local governments; changes in applicable legislation or application thereof; delays in obtaining approvals or financing or in the completion of development or construction activities; exchange rate fluctuations; requirements for additional capital; government regulation; environmental risks; reclamation expenses; outcomes of pending litigation; limitations on insurance coverage as well as those factors discussed in the Company’s other public disclosure documents, available on www.sedar.com. Although the Company has attempted to identify important factors that could cause actual results to differ materially from those contained in forward-looking statements, there may be other factors that cause results not to be as anticipated, estimated or intended. The Company believes that the expectations reflected in these forward?looking statements are reasonable, but no assurance can be given that these expectations will prove to be correct and such forward?looking statements included herein should not be unduly relied upon. These statements speak only as of the date hereof. The Company does not intend, and does not assume any obligation, to update these forward-looking statements, except as required by applicable laws.
Sources
Neither the Canadian
Securities Exchange nor the Market Regulator (as that term is defined in the
policies of the Canadian Securities Exchange) accepts responsibility for the
adequacy or accuracy of this release.
SOURCE: Element79 Gold Corp.

















