Could a Natural Mutation Slowdown COVID-19?
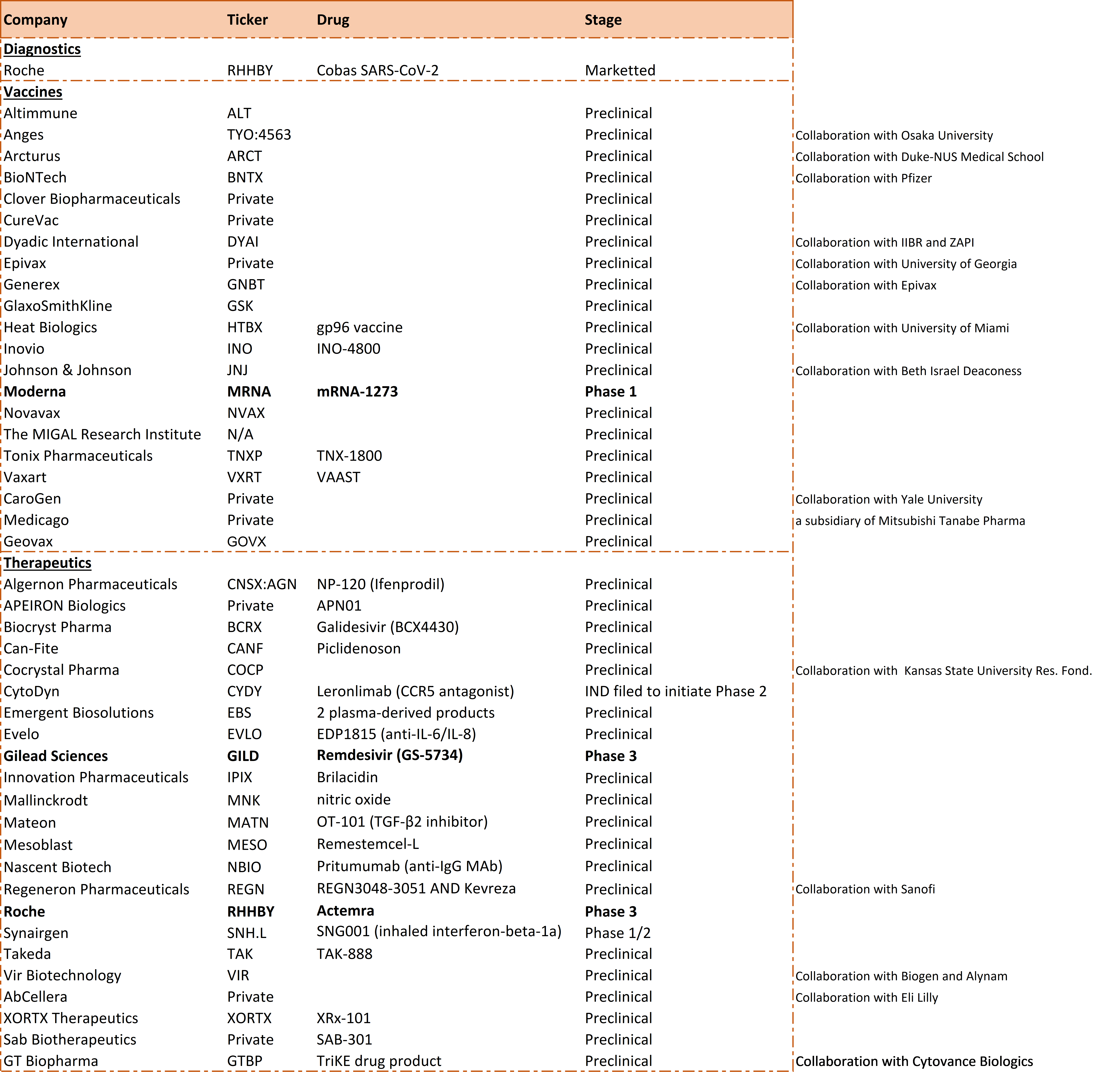
(Note: companies that
could be impacted by the content of this article are listed at the base of the
story [desktop version]. This article uses third-party references to provide a
bullish, bearish, and balanced point of view; sources are listed after the
Balanced section.)
“The
single biggest threat to man’s continued dominance on the planet is the virus”,
quote
by Joshua Lederberg (see photograph below).
The best medical scientists, physicians, epidemiologists, microbiologists, and infectious disease experts are currently joining forces worldwide to find a way to stop the spreading of SARS-CoV-2, the coronavirus causing “Coronavirus Disease of 2019” (Covid-19), which has become a global pandemic. As of March 22, 2020, there are more than 335,400 people infected with SARS-CoV-2 worldwide, and more than 14,610 people have died from the disease.
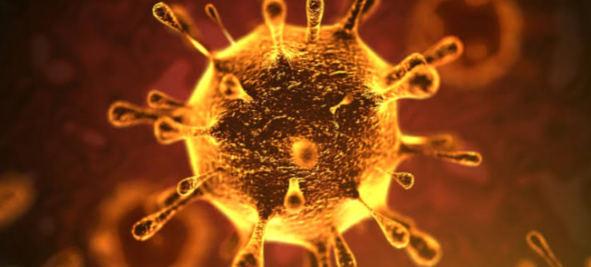
Figure
1 – Structure of the new coronavirus, SARS-CoV-2, images by transmission electron microscopy. The virus resembles an sphere with spikes. The S-protein or Spike protein is critical for virus entry into human cells.

Source
– Science 2020, 367 (6483) p1260-1263
As the biotechnology/pharmaceutical industry collaborates with medical academic institutions in the search for a treatment/vaccine or cure for Covid-19, it is difficult to predict the evolution of the current pandemic and virus behavior in coming weeks, months. As the world waits for the outcome of ongoing clinical trials evaluating potential treatments for Covid-19, it is important keeping aware of what medical science has taught us from previous epidemics such as SARS in 2002, middle east respiratory outbreak (MERS) in 2012 and Spanish flu (1918). Despite of the relatively short history of Covid-19, which started in China in December 2019, knowledge of the biology of the new coronavirus, SARSCoV-2, have grown very quickly.
New
Discoveries Could Speed up Vaccine Development
Recently, scientists at the University of Texas, Austin, discovered the mechanism of entry of SARS-CoV-2 into human cells (Science 2020, 367 (6483) p1260-1263). The Spike protein, or S-protein, is critical for viral entry. Another group of researchers led by Qiang Zhou, a research fellow at Westlake University in Hangzhou, China, have revealed how the new virus attaches to a receptor on respiratory cells called “angiotensin-converting enzyme 2”, or ACE2 (
Science March 4th, 2020: eabb2762 DOI: 10.1126/science.abb2762). Zhou and his team have now discovered the second part of the puzzle by describing how S-protein binds to ACE2 receptor on the surface of respiratory cells. This knowledge could be critical for the success of ongoing efforts to find a treatment or cure.
Figure
2 – Mechanism of entry of SARS-CoV-2 into human cells. The virus mechanism of entry is similar to the one utilized by the virus SARS-CoV, which caused the SARS outbreak of 2002. Panel A depicts “Spike protein” or “S-protein” on the surface of the coronavirus binding to “angiotensin-converting enzyme 2” (ACE-2) receptors on the surface of a human cell. Panel B shows the “type II transmembrane serine protease” (TMPRSS2) binding to and cleaving ACE-2 receptor, which activates the Spike protein of the coronavirus. Panel C shows viral entry.

Source
– Pathogens 2020, 9(3) 231; https://doi.org/10.3390/pathogens9030231
As the medical community races to find a treatment/cure for Covid-19, experts ponder on the probability that nature might help mankind by slowing down the virus spread. Among many potential mechanisms and strategies, there are three primary events which could curtail viral spreading:
- Natural Mutations of SARS-CoV-2
- Increasing Population Immunity
- Potential anti-viral treatments and Vaccines
The development of a drug treatment and/or effective vaccine will be a medical breakthrough which could save many lives worldwide. However, most experts believe testing of these candidate therapies will take time. As the SARS-CoV-2 virus continues to infect people across the globe, the number of patients with partial immunity could rise, which could eventually curtail viral spreading. However, the virus will probably has to infect many people worldwide before population immunity changes.
Could
a Mutation Reduce SARS-CoV-2 virulence?
(“Maybe
yes, Maybe not, Scientists say”)
The answer to this very important question is not that simple. Experts opinions are divided on this subject. According to Dr. Michael Farzan, a biologist from the Scripps Research Institute in Jupiter, Florida, who identified the structure of the Spike protein of SARS-CoV (Science 2005, 309(5742) p1864-8), SARS-CoV-2 will probably mutate to lose rather than gain virulence. There is the possibility that SARS-CoV-2 could become a seasonal endemic virus such as the influenza virus. Some scientists, including Dr. Farzan, believe the natural evolution of a virus selects for less virulent variants as they will transmit more efficiently. Their belief is that very sick patients are not very good at transmitting the disease, and dead people cannot do it at all. Thus a virus which kills quickly does not transmit infection very efficiently, and does not get naturally selected. In contrast, less virulent viruses transmit very well, as less sick patients act as a very efficient source of infection. In colloquial terms, “the virus wants to live, last longer in the infected population”.
Mutation
Allowing SARS-CoV-2 Jump from Bats to Humans – It is hypothesized that SARS-CoV-2 evolved through a mutation in the Spike protein allowing the virus to jump from bats to humans causing a zoonotic infection known as Covid-19. The Spike (S) protein of SARS-CoV-2 contains a “variable receptor-binding domain” (RBD), which binds to ACE-2 receptor in human cells facilitating viral cell entry (Figure 2). Using genomic DNA sequencing, scientists have identified a related virus, RaTG13, from bats (http://virological.org/t.the-proximal-origin-of-sars-cov-2/398). The hypothesis is that SARS-CoV-2 originated from bats, mutated, becoming able to infect human cells. A critical mutation of the virus allows human proteases to cut and activate the Spike protein of the virus, which facilitates viral entry and infection of human cells (Figure 2).
Mutations
Might Decrease Virulence – Some experts believe that in the same fashion the virus experienced this gain of function mutation, allowing cut and activation of Spike protein (Figure 2), increasing virulence, SARS-CoV-2 could also incur mutations causing a decrease in virulence. This was the case for the Spanish flu influenza virus, and for the coronavirus SARS-CoV which caused severe acute respiratory syndrome (SARS) in 2002 (Scientific Reports 2018, volume 8, article number: 15177). Mutated viruses became less virulent, viral spreading stopped, and the epidemics were halted as the viruses fizzle out. In theory, this could happen again with the virus causing Covid-19. Although infection with SARS-CoV-2 has shown a lower fatality rate than SARS-CoV, the new coronavirus SARS-CoV-2 is significantly more contagious. Differences aside, it is theoretically possible that the evolution of the current SARS-CoV-2 pandemic might end up in the same fashion as the SARS epidemic of 2002. In 2018, Dr. Christian Drosten, currently at German Center for Infectious Research in Berlin, published scientific work demonstrating that a variant of SARS-CoV lost part of its genome (29 nucleotides) during the SARS outbreak of 2002-2003, which made the SARS-CoV virus less virulent. The authors demonstrated this hypothesis by testing the SARS-CoV variant in cellular models in the laboratory (Scientific Reports 2018, volume 8, article number: 15177).
Other
Scientists Believe Mutations Will not Matter – Other experts are not too optimistic. Nature does not always help us, they think. Their belief is that natural mutations do very little to change the outcome of a pandemic. Most viral mutations do not elicit a new biological trait, which could be selected by natural selection. Indeed, most mutations are silent and do not change the outcome of a pandemic. All coronaviruses have shown high mutation rates. There is no reason to believe that SARS-CoV-2 will be any different in terms of mutation rate, despite of being more contagious than other viruses in the coronavirus family. Like SARS-CoV and MERS-CoV, the SARS-CoV-2 originated from an ancestor coronavirus harbored by bats. However, the intermediary host between bats and humans for SARS-CoV-2 has not been identified yet. Given the high mutation rates of these viruses, there is no certainty that a new mutation could cause loss of virulence as the opposite could also occur. Since the start of the pandemic, scientists have been sequencing strains of SARS-CoV-2 isolated from infected patients, and not a single mutation has shown to have an effect on virulence. The virus is very contagious as it is, and it might not need further mutations to be naturally selected. Dr. Andrew Rambaut, a molecular evolutionary biologist at the University of Edinburg, believes that mutations will not help to change virulence or alter the outcome of the current SARS-CoV-2 pandemic. Dr. Rambaut has been studying the genomes of SARS-CoV-2 isolates from patients. SARS-CoV-2 has 30,000 nucleotides. Dr. Rambaut mentioned that SARS-CoV-2 accumulates an average of one to two mutations per month, which although relatively high, it is still a slower mutation rate than what has been seen for the influenza virus. He believes most mutations do not affect the virus behavior.
Monitoring
SARS-CoV-2 Evolution
Monitoring the evolution of SARS-CoV-2 for mutations which could increase or decrease virulence is important (Pathogens 2020, 9, 186; doi:10.3390/pathogens9030186). Mutations in the gene encoding the Spike (S) protein do affect the virulence of SARS-CoV (New England Journal of Medicine 2003, 348 (20) p1948-1951). A very important statistic to monitor during the SARS-CoV-2 pandemic will be the “Basic Reproduction Number”, or “R0”, which measures the average number of people who will catch the disease from one infected person in a population who has never before being exposed to the disease (Emerg Infect Dis 2019, 25, 1-4). Thus far, the R0 for SARS-CoV-2 is estimated between 2 to 3 (Int J
Infect Dis 2020, 92, p214-217; New England Journal of Medicine 2020, doi:10.1056/NEJMoa2001316; Lancet 2020, doi:10.1016/S0140-6736(20)30260-9), which is relatively high. The goal is to reduce R0 to a number below 1.
Racing
to Develop a Vaccine
Moderna (Nasdaq:MRNA), in collaboration with the National Institute of Allergy and Infectious Diseases (NIH, NIAID), is developing mRNA-1273, a candidate vaccine targeting SARS-CoV-2. mRNA-1273 is designed to target the Spike (S) protein of the virus. mRNA vaccines function by encoding proteins, which once made by the cell stimulate the immune system to destroy infected cells. Development of mRNA vaccines is much faster than with traditional vaccines. mRNA-1273 encodes the “prefusion-stabilized” (Science 2020, 367 (6483) p1260-1263, DOI:10.1126/science.abb2507) form of Spike (S) protein of SARS-CoV-2. The candidate vaccine is currently in Phase I human clinical trials, which means it will take months before the effectiveness of this vaccine is known. Repurposing of existing anti-viral medicines is not an ideal strategy, but it could be a short term solution before an effective vaccine becomes available. For a full review of the pipeline of candidate medicines being developed by the biotechnology/pharmaceutical industry for SARS-CoV-2, see the journal Nature
Biotechnology published on March 20, 2020.
The
Singapore Story – Could a Mutation Partially Explain The City’s Success?
Although SARS-CoV2 genome seems to be more stable than the genomes of SARS-CoV and MERS-CoV, scientists have detected a SARS-CoV-2 variant containing a deletion of 382 nucleotides. This genetic variant of SARS-CoV-2 was isolated from eight infected patients in Singapore (“Discovery of a 382-nt deletion during the
early evolution of SARS-CoV-2” – bioRxiv preprint doi: https://doi.org/10.1101/2020.03.11.987222). The authors think the SARS-CoV-2 variant is less virulent, which may lead to a more attenuated phenotype of the virus, and less severe Covid-19 disease. A similar genetic deletion mutation, with attenuated phenotype, was observed in SARS-CoV during the SARS outbreak of 2002-2003. At present, the views on the relative success of Singapore dealing with Covid-19 (Singapore was one of the first cities with Covid-19 cases, but statistics are far better than other cities and countries around the world) is attributed to the government’s speed and efficiency dealing with the crisis. However, it is also plausible that Singapore was dealing with a less virulent variant of SARS-CoV-2.
Mankind definitely needs its biggest minds to focus on Covid-19 pandemic. Governments worldwide are working in concert to establish proper quarantine rules and other strict measures to slow the spreading of the disease. We hope new treatments and vaccines will be developed by the biotechnology/pharmaceutical industry. Patients are waiting, many lives could be saved by these efforts.
“The
single biggest threat to man’s continued dominance on the planet is the virus”,
quote
by Joshua Lederberg.
Joshua Lederberg (1925-2008) was an American molecular biologist known for his work in microbial genetics, artificial intelligence, and the United States space program. He was 33 years old when he won the 1958 Nobel Prize in Physiology or Medicine for contributions to the science of genetics.

Dr.
Joshua Lederberg (right) receiving the National Medal of Science from George
H.W. Bush
We need our heroes now, physicians and medical scientists who have dedicated their entire lives to save mankind from the threat of a virus.