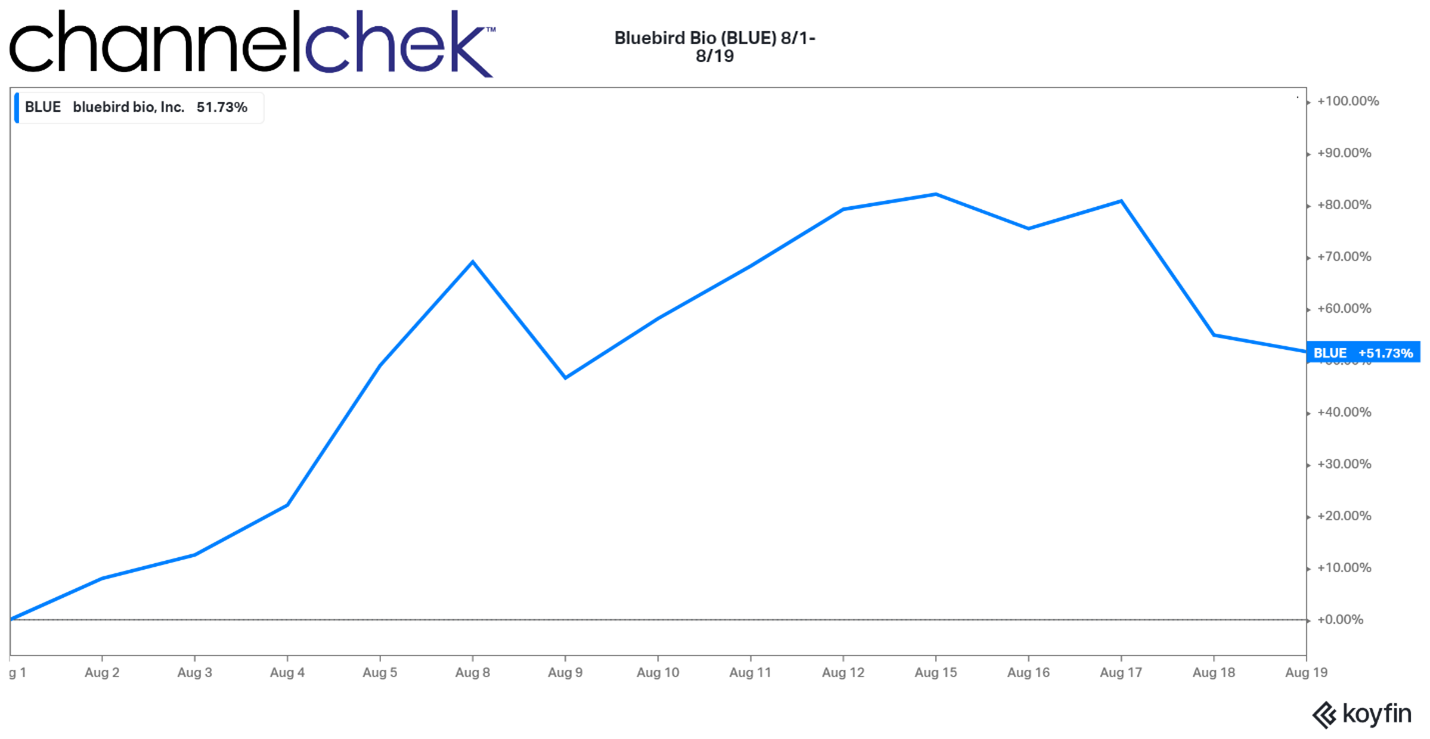
Tonix Pharmaceuticals Initiates Enrollment in Phase 2 PREVAIL Study of TNX-102 SL for the Treatment of Long COVID
Research, News, and Market Data on Tonix Pharmaceuticals
August 22, 2022 7:00am EDT
Results from Planned Interim Analysis Expected
First Half 2023
Long COVID Afflicts More Than 30% of Patients
Following Infection with SARS-CoV-2, the Virus that Causes COVID-19, and is
Expected to be a Global Health Burden
Concerning Rate of Opioid Use Observed in Long
COVID Patients with Multi-Site Pain
CHATHAM, N.J., Aug. 22, 2022 (GLOBE NEWSWIRE) — Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP) (Tonix or the Company), a clinical-stage biopharmaceutical company, today announced that the first participant was enrolled in the Phase 2 PREVAIL study of TNX-102 SL1 as a potential treatment for a subset of patients with Long COVID syndrome (Long COVID) whose symptoms overlap with fibromyalgia. Long COVID is known officially as Post-Acute Sequelae of COVID-19 (PASC)2.
“We are pleased to be starting a Phase 2 clinical study in this indication which is a growing problem and for which no drug is currently approved. We believe Long COVID, characterized by multi-site pain, fatigue and sleep disturbance, has features of central sensitization syndromes similar to fibromyalgia. Fibromyalgia is considered one of the clusters of chronic overlapping pain conditions that have much in common with Long COVID,” said Seth Lederman, M.D., Chief Executive Officer of Tonix Pharmaceuticals. “Findings from our retrospective observational database study in over 50,000 Long COVID patients showed that more than 40% of Long COVID patients in the sample have fibromyalgia-like multi-site pain symptoms, suggesting that we should be able to recruit a robust cohort of participants to test the effects of TNX-102 SL in treating this condition. Our experience with TNX-102 SL in fibromyalgia is the motivation for undertaking the development of TNX-102 SL in patients with Long COVID whose symptoms overlap with fibromyalgia. We recognize the need to better understand and develop treatment for Long COVID.”
“This retrospective observational database study also revealed the rate of opioid use in Long COVID patients,” said Greg Sullivan, M.D., Chief Medical Officer of Tonix Pharmaceuticals. “Opioid use was noted in 36% of Long COVID patients with multi-site pain symptoms relative to 19% of Long COVID patients without multi-site pain. In those with multi-site pain, opioid use increased to 39% of patients when fatigue was present, and 50% when insomnia was present. We look forward to continuing to progress this study with the goal of developing a non-addictive, centrally acting analgesic which potentially treats not only pain but also the sleep disturbance and fatigue that are common in Long COVID.”
About the Phase 2 PREVAIL
Study
The Phase 2 PREVAIL study is 14-week double-blind, randomized, multicenter, placebo-controlled study to evaluate the efficacy and safety of TNX-102 SL taken daily at bedtime in patients with multi-site pain associated with post-acute sequelae of SARS-COV-2 infection (PASC). The trial is being conducted at approximately 30 sites in the U.S. and is expected to enroll approximately 470 patients (235 per arm) who will be randomized in a 1:1 ratio to treatment with TNX-102 SL or placebo tablets. The primary efficacy endpoint will be changed from baseline in the weekly average of daily self-reported worst pain intensity scores at the Week 14 endpoint. Key secondary efficacy endpoints include change from baseline in self-reported scores for sleep disturbance, fatigue and cognitive function. An interim analysis is expected to be completed after the first 50% of enrolled patients have completed the study for the purpose of possible sample size re-estimation or to stop the study early for efficacy, currently anticipated in the first half of 2023.
For more information, see ClinicalTrials.gov Identifier: NCT05472090.
About Long COVID or
Post-Acute Sequelae of COVID-19 (PASC)
Although most people recover from COVID-19 within weeks of the acute illness, a substantial portion develop a chronic syndrome called Long COVID. These individuals experience a constellation of disabling symptoms long past the time of recovery from acute COVID-19. Most Long COVID patients who have been studied appear to have cleared the SARS-CoV-2 infection from their systems. The symptoms of Long COVID can include fatigue, sleep disorders, multi-site pain, fevers, shortness of breath, cognitive impairment described as “brain fog” or memory disturbance, gastrointestinal symptoms, anxiety, and depression. Long COVID can persist for many months and can range in severity from mild to incapacitating. Several cohort studies have reported that persistence of symptoms following SARS-CoV-2 infection occurs in more than 30% of patients.3-5 While typically associated with moderate or severe COVID-19, Long COVID can occur after mild COVID-19 or even after asymptomatic SARS-CoV-2 infection. Patients with Long COVID are sometimes referred to as “long-haulers”. Long COVID is a chronic disabling condition that is expected to result in a significant global health and economic burden.4-7 In response to the urgent need for therapies that address Long COVID, Congress awarded $1.15 billion to the National Institutes of Health to study Long COVID in December 2020.8 While the vaccines available in the U.S. through either FDA approval or under Emergency Use Authorization have been shown to prevent acute COVID, their ability to prevent Long COVID is unknown. There is currently no approved drug for the treatment of Long COVID.
1TNX-102 SL is an
investigational new drug and has not been approved for any indication.
2Feb. 24, 2021 – White
House COVID-19 Response Team press briefing; Feb 25, 2021 – policy brief from
the World Health Organization on long COVID.
3Harris, H, et
al. Tonix data on file. 2022
4Briggs, A, and Vassall,
A. (2021) “Count the cost of disability caused by COVID-19.” Nature
593(7860): 502-505.
5Nittas V, et al. (2022)
“Long COVID Through a Public Health Lens: An Umbrella Review.” Public
Health Rev. 43:1604501. Published 2022 Mar 15. doi:10.3389/phrs.2022.1604501
6Davis, HE., et al. (2021)
“Characterizing long COVID in an international cohort: 7 months of
symptoms and their impact.” EClinicalMedicine 38: 101019.
7Martin C, et al. (2021)
“A model framework for projecting the prevalence and impact of Long-COVID in
the UK.” PLoS One. 16(12):e0260843. Published 2021 Dec
2. doi:10.1371/journal.pone.0260843
8The NIH provision of
Title III Health and Human Services, Division M–Coronavirus Response and
Relief Supplemental Appropriations Act, 2021, of H.R. 133, The Consolidated
Appropriations Act of 2021. The bill was enacted into law on 27 December 2020,
becoming Public Law 116-260.
About TNX-102 SL
TNX-102 SL is a patented sublingual tablet formulation of cyclobenzaprine hydrochloride which provides rapid transmucosal absorption and reduced production of a long half-life active metabolite, norcyclobenzaprine, due to bypass of first-pass hepatic metabolism. As a multifunctional agent with potent binding and antagonist activities at the serotonin-5-HT2A, ?1-adrenergic, histaminergic-H1, and muscarinic-M1 receptors, TNX-102 SL is in clinical development as a daily bedtime treatment for Long COVID, fibromyalgia, PTSD, alcohol use disorder, and agitation in Alzheimer’s disease. The U.S. Patent and Trademark Office (USPTO) has issued United States Patent No. 9636408 in May 2017, Patent No. 9956188 in May 2018, Patent No. 10117936 in November 2018, Patent No. 10,357,465 in July 2019, and Patent No. 10736859 in August 2020. The Protectic™ protective eutectic and Angstro-Technology™ formulation claimed in these patents are important elements of Tonix’s proprietary TNX-102 SL composition. These patents are expected to provide TNX-102 SL, upon NDA approval, with U.S. market exclusivity until 2034/2035.
Tonix Pharmaceuticals
Holding Corp.*
Tonix is a clinical-stage biopharmaceutical company focused on discovering, licensing, acquiring and developing therapeutics to treat and prevent human disease and alleviate suffering. Tonix’s portfolio is composed of central nervous system (CNS), rare disease, immunology and infectious disease product candidates. Tonix’s CNS portfolio includes both small molecules and biologics to treat pain, neurologic, psychiatric and addiction conditions. Tonix’s lead CNS candidate, TNX-102 SL (cyclobenzaprine HCl sublingual tablet), is in mid-Phase 3 development for the management of fibromyalgia with a new Phase 3 study launched in the second quarter of 2022 and interim data expected in the first quarter of 2023. TNX-102 SL is also being developed to treat Long COVID, a chronic post-acute COVID-19 condition. Tonix initiated a Phase 2 study in Long COVID in the third quarter of 2022 and expects interim data in the first half of 2023. TNX-1300 (cocaine esterase) is a biologic designed to treat cocaine intoxication and has been granted Breakthrough Therapy designation by the FDA. A Phase 2 study of TNX-1300 is expected to be initiated in the fourth quarter of 2022. TNX-1900 (intranasal potentiated oxytocin), a small molecule in development for chronic migraine, is expected to enter the clinic with a Phase 2 study in the first half of 2023. Tonix’s rare disease portfolio includes TNX-2900 (intranasal potentiated oxytocin) for the treatment of Prader-Willi syndrome. TNX-2900 has been granted Orphan Drug designation by the FDA. Tonix’s immunology portfolio includes biologics to address organ transplant rejection, autoimmunity and cancer, including TNX-1500, which is a humanized monoclonal antibody targeting CD40-ligand (CD40L or CD154) being developed for the prevention of allograft and xenograft rejection and for the treatment of autoimmune diseases. A Phase 1 study of TNX-1500 is expected to be initiated in the first half of 2023. Tonix’s infectious disease pipeline consists of a vaccine in development to prevent smallpox and monkeypox, next-generation vaccines to prevent COVID-19, and a platform to make fully human monoclonal antibodies to treat COVID-19. TNX-801, Tonix’s vaccine in development to prevent smallpox and monkeypox, also serves as the live virus vaccine platform or recombinant pox vaccine (RPV) platform for other infectious diseases. A Phase 1 study of TNX-801 is expected to be initiated in Kenya in the first half of 2023. Tonix’s lead vaccine candidate for COVID-19 is TNX-1850, a live virus vaccines based on Tonix’s recombinant pox live virus vector vaccine platform. A Phase 1 study of the COVID-19 vaccine is expected to be initiated in the second half of 2023.
*All
of Tonix’s product candidates are investigational new drugs or
biologics and have not been approved for any indication.
This press release and further information about Tonix can be found at www.tonixpharma.com.
Forward Looking
Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking words such as “anticipate,” “believe,” “forecast,” “estimate,” “expect,” and “intend,” among others. These forward-looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, risks related to the failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID-19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. Tonix does not undertake an obligation to update or revise any forward-looking statement. Investors should read the risk factors set forth in the Annual Report on Form 10-K for the year ended December 31, 2021, as filed with the Securities and Exchange Commission (the “SEC”) on March 14, 2022, and periodic reports filed with the SEC on or after the date thereof. All of Tonix’s forward-looking statements are expressly qualified by all such risk factors and other cautionary statements. The information set forth herein speaks only as of the date thereof.
Contacts
Jessica Morris (corporate)
Tonix Pharmaceuticals
investor.relations@tonixpharma.com
(862) 904-8182
Olipriya Das, Ph.D. (media)
Russo Partners
Olipriya.Das@russopartnersllc.com
(646) 942-5588
Peter Vozzo (investors)
ICR Westwicke
peter.vozzo@westwicke.com
(443) 213-0505



Source: Tonix Pharmaceuticals Holding Corp.
Released
August 22, 2022