Research, News, and Market Data on SHWZ
November 9, 2022
OTCQX: SHWZ
NEO: SHWZ
Record Quarterly Revenue and Adjusted EBITDA
Revenue Increased 36% to $43.2 Million Compared to $31.8 Million in Q3 2021
Nine Month Revenue Increased 46% to $119.2 Million Compared to $81.9 Million
Adjusted EBITDA of $15.9 Million, 36.7% of Revenue
Nine Month Adjusted EBITDA of $38.7 Million, 32.5% of Revenue
Conference Call & Webcast Scheduled for Today – 5:00 pm EDT
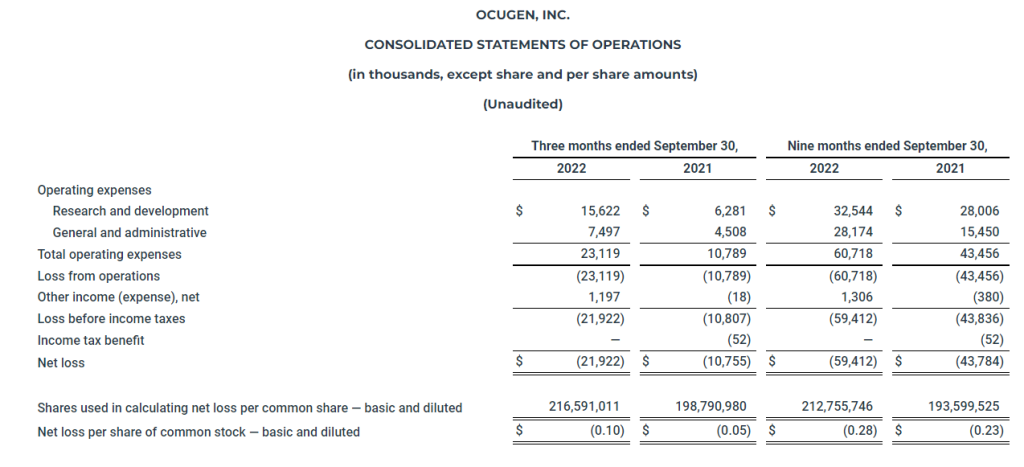
DENVER, Nov. 9, 2022 /CNW/ – Medicine Man Technologies Inc. operating as Schwazze, (OTCQX: SHWZ) (NEO: SHWZ) (“Schwazze” or the “Company”), today announced financial results for the third quarter ended September 30, 2022 (“Q3 2022”).
Q3 2022 Financial Summary:
- Revenues of $43.2 million increased 36% compared to $31.8 million in quarter ended September 30, 2021 (“Q3 2021”)
- Retail sales were $39.8 million up 92% to $20.7 million when compared to Q3 2021
- Gross Margin of $26.0 million, 60.1% of revenue, compared to $15.1 million and 47.3% of revenue in Q3 2021
- Net Income was $1.8 million compared to a Net Income of $1.0 million for the same period last year
- Adjusted EBITDA of $15.9 million was 36.7% of revenue, compared to $8.8 million for the same period last year
- Colorado two year stacked IDs for Q3 2022 compared to Q3 2022 and Q3 2020 for same store sales(1) were (9.7%) and one year IDs(1) were (10.6%) comparing Q3 2022 to Q3 2021
- Average basket size (1) for Q3 2022 was $60.96 up slightly by 0.1% compared to Q3 2021
- Recorded customer visits (1) for Q3 2022 totaled 452,220 down 10.7%, compared to Q3 2021
- New Mexico two year stacked IDs for Q3 2022 compared to Q3 2021 and Q3 2020 for same store sales(1) were 52.9% and one year IDs(1) were 48.4% comparing Q3 2022 to Q3 2021
- Average basket size (1) for Q3 2022 was $52.67 down 12.2% compared to Q3 2021
- Recorded customer visits (1) for Q3 2022 totaled 231,137 up 69.0%, compared to Q3 2021
Corporate Update:
Since December 2021, Schwazze has closed acquisitions adding 15 cannabis dispensaries, 10 in New Mexico and five in Colorado as well as four cultivation facilities in New Mexico and one in Colorado and one manufacturing asset in New Mexico. This year Schwazze has opened two new dispensaries in New Mexico. This brings our total dispensary count to 35 between Colorado and New Mexico.
Justin Dye, Chairman and CEO of Schwazze stated, “I am proud of the entire Schwazze team, and I would like to thank them for their hard work this past quarter and year. Despite a challenging economic backdrop, we outperformed our markets in Colorado by 12%. We’ve worked hard to continue to grow our market share, increase our profitability rate and generate free cash flow from operations, after paying taxes and CAPEX, placing us in an exclusive club within the cannabis sector. This is a proof point that we are well on our way to building Schwazze into a unique regional powerhouse. I believe our distinctive operating capabilities, applied to attractive growth opportunities within our sector, will reward our shareholders with attractive risk adjusted returns. The potential of favorable regulatory reform in the near-term would obviously accelerate and amplify those returns.”
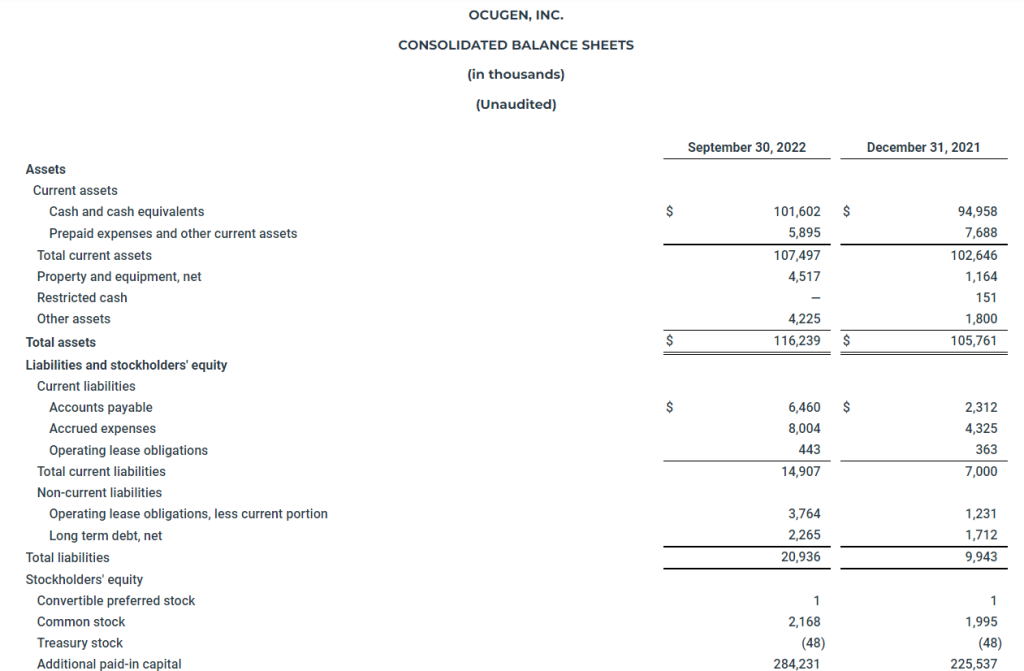
Q3 2022 Revenue
Revenues for the three months ended September 30, 2022 totaled $43,190,986, including (i) retail sales of $39,759,734 (ii) wholesale sales of $3,335,252 and (iii) other operating revenues of $96,000, compared to revenues of $31,835,305, including (i) retail sales of $20,741,864, (ii) wholesale sales of $11,022,519, and (iii) other operating revenues of $70,922 during the three months ended September 30, 2021, representing an increase of $11,355,681 or 36%. The most influential factor driving revenue increases in the third quarter of 2022 as compared to the same period in 2021 is acquisition activity. Revenue for the quarter ended September 30, 2022 included revenue from four consummated acquisitions in Colorado and revenue from the Company’s initial entrance into the New Mexico market with the acquisition of R. Greenleaf, which were not in revenue for the same period in 2021. Revenue from wholesale sales decreased, due in large part to continued pricing pressure in the Colorado wholesale market as a result of supply saturation in flower and bulk distillate products.
Cost of goods and services for the three months ended September 30, 2022, totaled $17,226,451 compared to cost of goods and services of $16,779,313 during the three months ended September 30, 2021, representing an increase of $447,138 or 3%. Overall cost of goods and services increased due to the same acquisition activities that generated substantial increases in revenue, but the rate at which cost of goods and services increases from acquisition activity occurs at a lower rate than increases in revenue from acquisition activity due to lower wholesale flower pricing in Colorado and substantial vertical integration in New Mexico and increased retail revenue, which has better gross margin, as a percentage of the total revenue.
Gross profit was $25,964,535 million dollars for the quarter compared to $15,055,992 during the same period in 2021. Gross profit margin increased as a percentage of revenue from 47.3% to 60.1%. This positive result reflects a higher percentage of retail sales, our consolidated purchasing approach, the implementation of our retail playbook, and vertical product sales in New Mexico.
Operating expenses for the quarter, totaled $14,849,677, compared to operating expenses of $11,218,992 during the same quarter 2021, representing an increase of $3,630,685 or 32%. This increase is due to increased selling, general and administrative expenses, professional service fees, salaries, benefits and related employment costs driven by growth from acquisitions offset by stock-based compensation.
Other expense, net for the three months ended September 30, 2022 totaled $3,712,108 compared to $1,555,427 during the three months ended September 30, 2021, representing an increase in other expense of $2,156,681 or 139%. The increase in other expenses is due to higher interest payments due on the Company’s debt obligations as a result of compounding interest with the passage of time and higher debt balances, which was partially offset this quarter by the revaluation of the derivative liability related to the Investor Notes issued in December 2021 that was recognized as income in the three months ended September 30, 2022.
Adjusted EBITDA for Q3 2022 was $15,860,466 representing 36.7% of revenue, compared to $8,797,641 and 27.6% of revenue for the same period last year. This is derived from Operating Income and adjusting one-time expenses, merger and acquisition and capital raising costs, non-cash related compensation costs, and depreciation and amortization. See the financial table for Adjusted EBITDA below adjustment for details.
For nine months ending September 30, 2022, the Company used cash for operations of $3,957,263 compared to generating cash of $4,814,104 for the same period in 2021. The Company has cash and cash equivalents of $38.7 million at the end of Q3 2022.
Nancy Huber, CFO for Schwazze commented, “During the third quarter we continued our focus on reducing operating and SG&A expenses. Our third quarter gross margin and operating expenses improved over the second quarter in both dollars and percent of revenue. Our balance sheet remains strong, with ample liquidity. We continue to be committed to delivering positive cash flow before acquisition costs for the year while driving organic growth with the opening of two stores in New Mexico in the third quarter.”
2022 Guidance
The Company is providing guidance for the fiscal year. FY 2022 revenue is projected to be $155 million to $165 million, and the FY 2022 adjusted EBITDA is projected to be from $51 million to $56 million. We are on target to deliver the lower end of the range for adjusted EBITDA which was a fourth quarter annualized run-rate of $60-72 million dollars. We expect to be slightly below the projected revenues which was a fourth quarter annualized run-rate of $175 million to $200 million. This lower-than-expected revenue in Q4 is due to lower than expected wholesale sales, and construction delays in new store openings in New Mexico.
The company generated $4 million in cash from operations in the third quarter and expects to generate positive cash flow before acquisitions for the year.
| NOTES: | |
| (1) | Schwazze did not own all the assets and entities in part of 2021, 2020 and 2019 and is using unaudited numbers for this comparison. |
Adjusted EBITDA represents income (loss) from operations, as reported, before tax, adjusted to exclude non-recurring items, other non-cash items, including stock-based compensation expense, depreciation, and amortization, and further adjusted to remove acquisition and capital raise related costs, and other one-time expenses, such as severance, retention, and employee relocation. The Company uses adjusted EBITDA as it believes it better explains the results of its core business. The Company has not reconciled guidance for adjusted EBITDA to the corresponding GAAP financial measure because it cannot provide guidance for the various reconciling items. The Company is unable to provide guidance for these reconciling items because it cannot determine their probable significance, as certain items are outside of its control and cannot be reasonably predicted. Accordingly, a reconciliation to the corresponding GAAP financial measure is not available without unreasonable effort.
Webcast – November 9, 2022 – 5:00 PM EDT
Investors and stakeholders may participate in the conference call by dialing 416-764-8650 or by dialing North American toll free 1-888-664-6383 or listen to the webcast from the Company’s website at https://ir.schwazze.com The webcast will be available on the Company’s website and on replay until November 16, 2022, and may be accessed by dialing 1-888-390-0541 / 997573 #.
Following their prepared remarks, Chief Executive Officer, Justin Dye; President, Nirup Krishnamurthy; and Chief Financial Officer, Nancy Huber will answer investor questions. Investors may submit questions in advance or during the conference call itself through the weblink: https://app.webinar.net/x0q6rpnP84n. This weblink has been posted to the Company’s website and will be archived on the website. All Company SEC filings can also be accessed on the Company website at https://ir.schwazze.com/sec-filings
About Schwazze
Schwazze (OTCQX: SHWZ, NEO: SHWZ) is building a premier vertically integrated regional cannabis company with assets in Colorado and New Mexico and will continue to take its operating system to other states where it can develop a differentiated regional leadership position. Schwazze is the parent company of a portfolio of leading cannabis businesses and brands spanning seed to sale. The Company is committed to unlocking the full potential of the cannabis plant to improve the human condition. Schwazze is anchored by a high-performance culture that combines customer-centric thinking and data science to test, measure, and drive decisions and outcomes. The Company’s leadership team has deep expertise in retailing, wholesaling, and building consumer brands at Fortune 500 companies as well as in the cannabis sector. Schwazze is passionate about making a difference in our communities, promoting diversity and inclusion, and doing our part to incorporate climate-conscious practices. Medicine Man Technologies, Inc. was Schwazze’s former operating trade name. The corporate entity continues to be named Medicine Man Technologies, Inc. Schwazze derives its name from the pruning technique of a cannabis plant to enhance plant structure and promote healthy growth.
Forward-Looking Statements
Such forward-looking statements may be preceded by the words “plan,” “will,” “may,” “continue,” “anticipate,” “become,” “build,” “develop,” “expect,” “believe,” “poised,” “project,” “approximate,” “could,” “potential,” or similar expressions as they relate to Schwazze. Forward-looking statements include the guidance provided regarding the Company’s Q4 2022 performance and annual capital spending. Forward-looking statements are not guarantees of future events or performance, are based on certain assumptions, and are subject to various known and unknown risks and uncertainties, many of which are beyond the Company’s control and cannot be predicted or quantified. Consequently, actual events and results may differ materially from those expressed or implied by such forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties associated with (i) our inability to manufacture our products and product candidates on a commercial scale on our own or in collaboration with third parties; (ii) difficulties in obtaining financing on commercially reasonable terms; (iii) changes in the size and nature of our competition; (iv) loss of one or more key executives or scientists; (v) difficulties in securing regulatory approval to market our products and product candidates; (vi) our ability to successfully execute our growth strategy in Colorado and New Mexico and outside the states, (vii) our ability to identify and consummate future acquisitions that meet our criteria, (viii) our ability to successfully integrate acquired businesses and realize synergies therefrom, (ix) the ongoing COVID-19 pandemic, * the timing and extent of governmental stimulus programs, (xi) the uncertainty in the application of federal, state and local laws to our business, and any changes in such laws, and (xii) our ability to achieve the target metrics, including our annualized revenue and EBIDTA run rates set out in our Q4 2022 guidance. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company’s filings with the Securities and Exchange Commission (SEC), including the Company’s Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q. Investors and security holders are urged to read these documents free of charge on the SEC’s website at http://www.sec.gov. The Company assumes no obligation to publicly update or revise its forward-looking statements as a result of new information, future events or otherwise except as required by law.
View original content to download multimedia:https://www.prnewswire.com/news-releases/schwazze-announces-third-quarter-results-301673612.html
SOURCE Medicine Man Technologies, Inc.