Chakana Reports 50.7m Of 0.63 g/t Gold, 3.46% Copper And 118.8 g/t Silver (4.89% Cu-Eq) In Huancarama At Soledad, Peru
Research, News, and Market Data on Chakana Copper
Soledad Project Highlights Include:
- Remaining 13 resource definition and exploration holes at Huancarama reported totalling 3,265m
- Two additional high-grade intervals of >50m lengths also reported
- 56.15 m @ 0.45 g/t Au, 115.1 g/t Ag, and 2.34% Cu (3.62% Cu-EQ)
- 54.90 m @ 0.63 g/t Au, 81.7 g/t Ag, and 2.41% Cu (3.52% Cu-EQ)
- Off-set IP surveys continue over high priority targets defined by gradient array and other data sets; numerous new targets have been defined
- Finalizing first-ever resource estimate on the Soledad Project
- 16 out of 110 current targets have been tested to date (15%)
Vancouver, B.C., January 4, 2022 – Chakana Copper Corp. (TSX-V: PERU; OTCQB: CHKKF; FRA: 1ZX) (the “Company” or “Chakana”), is pleased to provide results from thirteen additional resource definition and exploration holes drilled in Huancarama totaling 3,265m at the Soledad project, Ancash, Peru (see table below). The resource drilling is part of a fully funded exploration and resource drilling program completed in 2021 (Fig. 1). These results compliment previous results from Huancarama and will increase confidence in the initial resource estimate covering several breccia pipes, which is currently being finalized.
“The multiple high-grade intercepts from Huancarama are a fitting conclusion to the 2020-2021 resource drilling program at Soledad. Since restarting the drill program in August of 2020, we have completed over 30,000m of drilling in 143 drill holes. The initial resource estimate on the shallower extent of several breccia pipes, a first for the project, is nearing completion and will help us better understand the upside potential of the broader Soledad project. Mineralized tourmaline breccia pipes occur within a 12 km2 area, within which we have defined 110 targets through systematic multidisciplinary exploration. Only 16 out of the 110 targets have been tested thus far. In addition, the current geophysical program has defined numerous new targets within the known productive structural corridors that host mineralized breccia pipes,” stated President and CEO David Kelley.
Results
Huancarama (Resource Definition)
| DDH # |
FROM – TO (M) |
CORE
LENGTH
(M) |
AU
G/T |
AG
G/T |
CU
% |
CU-EQ
%* |
AU-EQ
G/T* |
| SDH21-254 |
81.20 |
154.00 |
72.80 |
0.98 |
87.4 |
0.53 |
1.93 |
2.89 |
| including |
125.00 |
145.00 |
20.00 |
2.61 |
222.3 |
1.06 |
4.67 |
7.14 |
| and |
181.00 |
199.00 |
18.00 |
0.73 |
47.3 |
0.19 |
1.07 |
1.64 |
| SDH21-257 |
71.00 |
82.00 |
11.00 |
0.34 |
64.8 |
0.18 |
0.96 |
1.46 |
| and |
90.00 |
103.00 |
13.00 |
0.26 |
65.5 |
0.22 |
0.95 |
1.45 |
| and |
124.00 |
166.00 |
42.00 |
0.31 |
34.9 |
0.77 |
1.27 |
1.94 |
| SDH21-259 |
92.00 |
99.00 |
7.00 |
1.08 |
333.9 |
1.92 |
5.48 |
8.38 |
| SDH21-263 |
155.00 |
196.00 |
41.00 |
0.52 |
32.0 |
0.51 |
1.12 |
1.72 |
| SDH21-265 |
74.00 |
168.00 |
94.00 |
0.37 |
45.9 |
0.82 |
1.45 |
2.22 |
| including |
102.00 |
119.00 |
17.00 |
0.80 |
76.6 |
1.90 |
3.08 |
4.71 |
| SDH21-266 |
54.00 |
161.85 |
107.85 |
0.46 |
62.7 |
0.72 |
1.56 |
2.38 |
| and |
169.00 |
173.00 |
4.00 |
1.39 |
281.0 |
7.38 |
10.69 |
16.35 |
| SDH21-267 |
124.00 |
146.00 |
22.00 |
2.71 |
162.5 |
1.91 |
5.07 |
7.76 |
| SDH21-268 |
59.00 |
61.00 |
2.00 |
0.64 |
611.4 |
12.35 |
18.00 |
27.53 |
| and |
64.00 |
65.00 |
1.00 |
0.27 |
189.0 |
11.50 |
13.29 |
20.33 |
| and |
72.00 |
172.00 |
100.00 |
0.36 |
39.9 |
0.63 |
1.21 |
1.85 |
| SDH21-269 |
79.00 |
143.00 |
64.00 |
0.46 |
32.2 |
0.64 |
1.22 |
1.86 |
| SDH21-271 |
168.30 |
219.00 |
50.70 |
0.63 |
118.8 |
3.46 |
4.89 |
7.48 |
| including |
168.30 |
189.00 |
20.70 |
1.12 |
181.8 |
6.64 |
8.93 |
13.65 |
| SDH21-273 |
170.85 |
227.00 |
56.15 |
0.45 |
115.1 |
2.34 |
3.62 |
5.53 |
| including |
173.00 |
180.00 |
7.00 |
1.02 |
393.7 |
9.17 |
13.20 |
20.20 |
| and |
274.00 |
281.00 |
7.00 |
1.12 |
92.2 |
2.22 |
3.74 |
5.72 |
| SDH21-275 |
43.00 |
47.10 |
4.10 |
0.67 |
91.2 |
0.17 |
1.39 |
2.12 |
| and |
167.10 |
222.00 |
54.90 |
0.63 |
81.7 |
2.41 |
3.52 |
5.38 |
| including |
167.10 |
177.00 |
9.90 |
1.62 |
230.4 |
5.93 |
8.96 |
13.70 |
| and |
277.00 |
285.00 |
8.00 |
0.40 |
31.4 |
1.03 |
1.56 |
2.39 |
* Cu_eq and Au_eq values were calculated using copper, gold, and silver. Metal prices utilized for the calculations are Cu – US$2.90/lb, Au – US$1,300/oz, and Ag – US$17/oz. No adjustments were made for recovery as the project is an early-stage exploration project and metallurgical data to allow for estimation of recoveries are not yet available. The formulas utilized to calculate equivalent values are Cu-eq (%) = Cu% + (Au g/t * 0.6556) + (Ag g/t * 0.00857) and Au-eq (g/t) = Au g/t + (Cu% * 1.5296) + (Ag g/t * 0.01307).
Huancarama
The Huancarama breccia pipe is in the central part of the project at an elevation of 3,950m and is one of several breccia pipes that will be included in Chakana’s initial resource estimate (Fig. 1). This breccia pipe is part of a breccia complex with six outcropping breccias over a lateral distance of 200m east-west. Two of the breccias, separated by 50m at surface, coalesce at depth, forming a larger breccia pipe approximately 100m x 60m in plan. Breccia has been intercepted to a depth of 492m below surface and remains open.
Drill holes described in this release were drilled from three different platforms and were designed to confirm the geometry and continuity of mineralization within the breccia pipe (Figs. 2 and 3). All resource definition holes intersected significant mineralization (see Figure 4 for select core photos of the mineralization). In addition to the high copper, gold and silver grades reported in these drill holes, three of the holes also intersected very strong molybdenum mineralization at depts of around 350m below surface (Fig. 3). For example, SDH21-271 intersected 36m of 0.26% molybdenum from 337m; SDH21-273 intersected 13.7m of 0.29% molybdenum from 354m; and SDH21-275 intersected 21.25m of 0.20% molybdenum from 350m. The significance of this mineralization in relation to surrounding intrusive rocks is being investigated.
One exploration hole (SDH21-274) was drilled to the northeast of the main Huancarama breccia complex testing a possible feeder structure to the breccia complex. This hole intersected strongly altered andesitic lithic tuff along structures, but no significant mineralization was encountered.
2021 Resource and Exploration Drill Program
A total of 23,947m of drilling has been completed in 2021. The objectives of this drill program are to complete resource definition drilling on several breccia pipes and test several new exploration targets. Breccia pipes that will be included in the initial resource estimate are: Bx1, Bx 5, Bx6, Paloma East, Paloma West, and Huancarama (Fig. 1); a seventh pipe, Bx 7, may also be included pending a final evaluation of the results to date.
During 2021 our drilling was focused on the north half of the project where drill permits are in place. Permitting for the south half of the project is well advanced. The southern half of the property hosts several outcropping mineralized tourmaline breccia pipes and has been recently covered by the Company’s ongoing geophysical program. Numerous targets exist, none of which have been drilled previously.
Geophysical Surveys
Gradient-array induced-polarization (IP) surveys have been completed over the entire 12km2 footprint of the Soledad mineral system. Off-set IP surveys are now in-progress covering high priority target areas. This work complements the extensive exploration database that supports our current inventory of 110 exploration targets. This new information identifies both new targets and prioritizes existing targets that will be tested when the exploration drilling programs resume.
About Chakana Copper
Chakana Copper Corp is a Canadian-based minerals exploration company that is currently advancing the Soledad Project located in the Ancash region of Peru, a highly favorable mining jurisdiction with supportive communities. The Soledad Project is notable for the high-grade copper-gold-silver mineralization that is hosted in tourmaline breccia pipes. A total of 60,854 metres in 260 diamond core holes for exploration and resource definition drilling have been completed since 2017, testing 16 of 110 total exploration targets, confirming that Soledad is a large, well-endowed mineral system with strong exploration upside. Chakana’s investors are uniquely positioned as the Soledad Project provides exposure to base and precious metals. For more information on the Soledad project, please visit the website at www.chakanacopper.com.
Sampling and Analytical Procedures
Chakana follows rigorous sampling and analytical protocols that meet or exceed industry standards. Core samples are stored in a secured area until transport in batches to the ALS facility in Callao, Lima, Peru. Sample batches include certified reference materials, blank, and duplicate samples that are then processed under the control of ALS. All samples are analyzed using the ME-MS41 (ICP technique that provides a comprehensive multi-element overview of the rock geochemistry), while gold is analyzed by AA24 and GRA22 when values exceed 10 g/t by AA24. Over limit silver, copper, lead and zinc are analyzed using the OG-46 procedure. Soil samples are analyzed by 4-acid (ME-MS61) and for gold by Fire Assay on a 30g sample (Au-ICP21).
Results of previous drilling and additional information concerning the Project, including a technical report prepared in accordance with National Instrument 43-101, are made available on Chakana’s SEDAR profile at www.sedar.com.
Qualified Person
David Kelley, an officer, and a director of Chakana, and a Qualified Person as defined by NI 43-101, reviewed and approved the technical information in this news release.
ON BEHALF OF THE BOARD
(signed) “David Kelley”
David Kelley
President and CEO
For further information contact:
Joanne Jobin, Investor Relations Officer
Phone: 647 964 0292
Email: jjobin@chakanacopper.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward-looking Statement Advisory: This release may contain forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties, and other factors which may cause the actual results, performance, or achievements of Chakana to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statements. Forward looking statements or information relates to, among other things, the interpretation of the nature of the mineralization at the Soledad copper-gold-silver project (the “Project”), the potential to expand the mineralization, and to develop and grow a resource within the Project, the planning for further exploration work, the ability to de-risk the potential exploration targets, and our belief in the potential for mineralization within unexplored parts of the Project. These forward-looking statements are based on management’s current expectations and beliefs but given the uncertainties, assumptions and risks, readers are cautioned not to place undue reliance on such forward- looking statements or information. The Company disclaims any obligation to update, or to publicly announce, any such statements, events or developments except as required by law.

Figure 1 – View looking north showing outcropping breccia pipes and occurrences within the northern Soledad cluster. Pipes that will be included in the initial resource are shown in green (Bx 1, Bx 5, Bx 6, Paloma East, Paloma West, and Huancarama). Breccia pipes shown in yellow have had exploration drilling completed. Other pipes/occurrences and targets defined by other exploration data remain to be tested by drilling. Additional breccia pipes occur on the south half of the property and are not shown here.
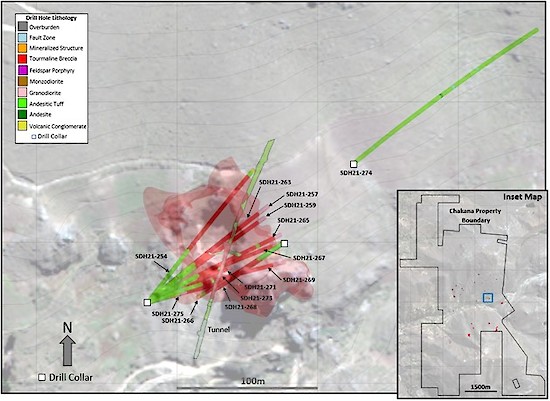
Figure 2 – Map showing drill holes reported in this release and modeled breccia pipes at Huancarama (light red shape) based on all drill holes. Light gray contours are at 10m intervals. Blue rectangle in the inset map shows the area of Figure 2 within the overall Soledad property.

Figure 3 – 3D sectional view of Huancarama looking west-northwest. Light red 3D shape shows breccia pipe geometry based on all drill holes. High molybdenum zone intersected in holes SDH21-271, SDH21-273, and SDH21-275 highlighted.

Figure 4 – Select core photos from Huancarama reported in this release: SDH21-257 (125.0m) tourmaline breccia with chalcopyrite-pyrite replacing clasts; SDH21-259 (96.9m) chalcopyrite-pyrite filling open cavity in breccia; SDH21-269 (105.2m) tourmaline breccia with chalcopyrite-pyrite replacing clasts; SDH21-271 (170.85m) chalcopyrite-cemented tourmaline breccia; SDH21-271 (184.85m) chalcopyrite-cemented tourmaline breccia; SDH21-273 (173.0m) chalcopyrite-cemented tourmaline breccia; SDH21-275 (183.3m) massive chalcopyrite-pyrite filling void in breccia with siderite. Core diameter is 6.35cm (HQ) in all instances.