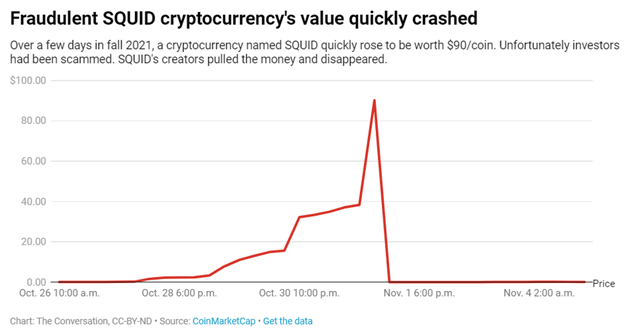
Tonix Pharmaceuticals Announces
Publication of Paper in Pharmaceutics on the Enhancing Effect That Magnesium
Contributes to in vivo Intranasal Oxytocin Analgesia
June 21, 2022 7:00am EDT
Intranasal Oxytocin with
Magnesium Demonstrated Augmented Craniofacial Analgesia in an Animal Model
Enhanced Effect of Mg2+ is the Core Patented Technology of TNX-1900 for Migraine
TNX-2900 Orphan Drug Designated
Product for Prader-Willi Syndrome Also Contains Mg2+
Issued Patents Expected to
Provide Exclusivity Until 2036
CHATHAM, N.J., June 21, 2022 (GLOBE NEWSWIRE) — Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP), a clinical-stage biopharmaceutical company, announced the publication of a paper, entitled “Impact of Magnesium on Oxytocin Receptor Function,” in the journal
Pharmaceutics, that described results from a research team led by Professor David Yeomans1. The paper includes data showing the enhancing effects of magnesium (Mg2+) on the activity of intranasal oxytocin in an animal model of craniofacial pain. The Mg2+ enhanced formulation of intranasal oxytocin is the basis for the Company’s TNX-19002 drug candidate in development to prevent migraine headaches in chronic migraineurs, and TNX-29002 which is in development to treat hyperphagia (over-eating) in adolescent and young adult patients with Prader-Willi syndrome. Professor Yeomans was the scientific founder of Trigemina, Inc. from which Tonix acquired rights to the Mg2+enhanced oxytocin technology. Professor Yeomans is a consultant to Tonix and the research described in the paper was funded in part by Tonix.
“This new paper further evidences the important role Mg2+ plays in the effect of oxytocin on pain reduction both in vitro and in vivo in an animal model of head pain,” said Seth Lederman, M.D., Chief Executive Officer of Tonix Pharmaceuticals. “Prior to this work, studies of intranasal oxytocin on pain yielded inconsistent results because the analgesic effect of oxytocin decreased with higher doses, a phenomenon called ‘high dose inhibition’ or colloquially, as an ‘inverted U’ shaped dose response. Professor Yeomans and his team have shown that adding Mg2+ to intranasal oxytocin reduces or eliminates the ‘high dose inhibition’, such that analgesia increases with higher doses of oxytocin. Consequently, we expect that the Mg2+ enhanced formulation of intranasal oxytocin in TNX-1900 and TNX-2900 may provide consistent effects to the extent that the Mg2+ component of the formulation reduces or eliminates the high dose inhibition and may also allow for using higher oxytocin doses.”
Professor Yeomans said, “Mg2+ levels modulate the affinity of oxytocin receptor for oxytocin
in
vitro. We have now confirmed that at above physiological concentrations Mg2+ increases the activity of oxytocin for oxytocin receptor in vivo. Intranasal oxytocin with Mg2+ demonstrated augmented craniofacial analgesia in animal models. Critically, we have also demonstrated that, under test conditions, oxytocin has an ‘inverted U’ shaped dose response which Mg2+ can overcome, potentially allowing for the use of higher oxytocin doses. The potential clinical significance of these observations is that the formulation of oxytocin plus Mg2+ in Tonix’s TNX-1900 and TNX-2900 has the potential to enhance oxytocin efficacy for pain as well as for other uses.”
“Tonix is excited to develop Mg2+-enhanced formulation of intranasal oxytocin as a non-addictive treatment for migraine and craniofacial pain and for the treatment of Prader-Willi Syndrome,” added Dr. Lederman. “TNX-1900 will enter Phase 2 for prophylaxis of chronic migraine in the second half of 2022 and TNX-2900 is in development for treating hyperphagia in Prader Willi syndrome. The preclinical data that we have seen to date are promising and show that oxytocin, a natural hormone, is capable of blocking the release of the neurotransmitter calcitonin gene-related peptide (CGRP) in the brain coverings and trigeminal ganglia, thus potentially modulating a key step in the causation of migraine. It has been shown that intranasally delivered oxytocin selectively reaches the trigeminal ganglia with low systemic absorption. Overall, we believe that TNX-1900 has the potential to be a non-addicting, non-constipating and easy to administer alternative to opioids to treat migraine and craniofacial pain. We believe targeted delivery of oxytocin could translate into selective blockade of CGRP release in the trigeminal ganglion and not throughout the body, which could be a potential safety advantage over systemic CGRP inhibition. In addition, daily dosing is more quickly reversible, in contrast to monthly or quarterly dosing, giving physicians and their patients greater control.”
About Intranasal Oxytocin
Oxytocin is a naturally occurring human hormone that acts as a neurotransmitter in the brain. Oxytocin has no recognized addiction potential. Oxytocin is approved by the U.S. Food and Drug Administration (FDA) as Pitocin®3, an intravenous infusion or intramuscular injection drug, for use in pregnant women to induce labor. An intranasal form of oxytocin was marketed in the U.S. by Novartis to assist in the production of breast milk as Syntocinon®4 (oxytocin nasal 40 units/ml), but the product was discontinued, and the New Drug Application (NDA) has been withdrawn.
About Migraine
Migraine is a neurological condition that manifests in throbbing headache, often on one side of the head, that lasts at least four hours. It can also be accompanied by nausea, vomiting, visual disturbances, and sensitivity to bright light, strong smells, and loud noises5. Epidemiological studies indicate that globally, approximately 1.2 billion individuals suffer from migraines annually.6 Approximately 39 million Americans suffer from migraines and among these individuals, approximately four million experience chronic migraines (15 or more headache days per month).6
About TNX-19002
TNX-1900 (intranasal potentiated oxytocin) is a proprietary formulation of oxytocin and Mg2+ in development as a candidate for prophylaxis of chronic migraine and for the treatment of craniofacial pain, insulin resistance and related conditions. In 2020, TNX-1900 was acquired from Trigemina, Inc. TNX-1900 is a drug-device combination product, based on an intranasal actuator device that delivers oxytocin and Mg2+ into the nose. It has been observed that low oxytocin levels in the body can lead to increase in migraine headache frequency, and that increased oxytocin levels can relieve migraine headaches. Certain other chronic pain conditions are also associated with decreased oxytocin levels. Migraine attacks are caused, in part, by the activity of pain-sensing trigeminal nerve cells which, when activated, release of CGRP which binds to receptors on other nerve cells and starts a cascade of events that is believed to result in headache. Oxytocin when delivered via the nasal route, concentrates in the trigeminal system8 resulting in binding of oxytocin to receptors on neurons in the trigeminal system, inhibiting transmission of pain signals and the release of CGRP.9 Blocking CGRP release is a distinct mechanism compared with CGRP antagonist and anti-CGRP antibody drugs, which block the binding of CGRP to its receptor. With TNX-1900, the addition of magnesium to the oxytocin formulation enhances oxytocin receptor binding10 as well as its effects on trigeminal neurons and craniofacial analgesic effects in animal models1. Intranasal oxytocin has been well tolerated in several clinical trials in both adults and children
11. Targeted nasal delivery results in low systemic exposure and lower risk of non-nervous system, off-target effects which could potentially occur with systemic CGRP antagonists such as anti-CGRP antibodies12. For example, CGRP has roles in dilating blood vessels in response to ischemia, including in the heart. Tonix has licensed technology from the University of Geneva to use TNX-1900 for the treatment of insulin resistance and related conditions.
About Prader
Willi Syndrome
Prader-Willi syndrome is a rare genetic disorder of failure to thrive in infancy and uncontrolled appetite and obesity in childhood and adulthood with no approved treatments available that occurs in approximately one in 15,000 births. Prader-Willi syndrome results in physical, mental and behavioral problems. A key feature of Prader-Willi syndrome in infants is a lack of suckling and poor muscle strength which leads to malnutrition and failure to thrive. However, paradoxically in children and adults, the key feature of Prader-Willi syndrome is a constant sense of hunger (hyperphagia), which leads to severe obesity. Intranasal oxytocin improves suckling in newborn animals but also suppresses feeding behaviors in adult animal models.
About TNX-29005
TNX-2900 (intranasal potentiated oxytocin) is a proprietary formulation of oxytocin and Mg2+ in development as a candidate for treatment of hyperphagia in Prader-Willi syndrome. TNX-2900 is a drug-device combination product, based on an intranasal actuator device that delivers oxytocin and Mg2 into the nose. Tonix licensed technology to treat Prader Willi Syndrome and non-organic failure to thrive disease from Inserm (the French National Institute of Health and Medical Research). The licensing agreement was negotiated and signed by Inserm Transfert, the private subsidiary of Inserm, on behalf of Inserm, Aix-Marseille Université and Centre Hospitalier Universitaire of Toulouse. The co-exclusive license allows Tonix to expand its intranasal potentiated oxytocin development program to the treatment of Prader-Willi syndrome. The patents covering the technology are expected to provide market exclusivity for the co-licensees in the U.S. and Europe through 2031, which exclusivity could be extended after marketing authorization by a Supplemental Protection Certificate in Europe or a Patent Term Extension in the U.S., independent of other Tonix-held patents covering the formulation and oxytocin potentiation technologies for intranasal administration.
1Bharadwaj VN, et al., Pharmaceutics. 2022; 14(5):1105. https://doi.org/10.3390/pharmaceutics14051105
2TNX-1900 and TNX-2900 are investigational new drugs and have not been approved for any indication.
3Pitocin® is a trademark of Par Pharmaceutical, Inc.
4Syntocinon® is a trademark of BGP Products Operations GmbH.
5https://www.mayoclinic.org/diseases-conditions/migraine-headache/symptoms-causes/syc-20360201
6Burch et al., Migraine:
Epidemiology, Burden, and Comorbidity, Neurol Clin 37 (2019) 631–649.
7Yeomans, DC et al. 2017. US patent US2017368095.
8Yeomans DC, et al. Transl Psychiatry. 2021. 11(1):388.
9Tzabazis A, et al. Cephalalgia. 2016. 36(10):943-50.
10Antoni FA and Chadio SE. Biochem J. 1989. 257(2):611-4.
11Cai Q, et al., Psychiatry Clin Neurosci. 2018. Mar;72(3):140-151.
12MaassenVanDenBrink A, et al. Trends Pharmacol Sci. 2016. 37(9):779-788.
About Tonix
Pharmaceuticals Holding Corp.*
Tonix is a clinical-stage biopharmaceutical company focused on discovering, licensing, acquiring and developing therapeutics to treat and prevent human disease and alleviate suffering. Tonix’s portfolio is composed of central nervous system (CNS), rare disease, immunology and infectious disease product candidates. Tonix’s CNS portfolio includes both small molecules and biologics to treat pain, neurologic, psychiatric and addiction conditions. Tonix’s lead CNS candidate, TNX-102 SL (cyclobenzaprine HCl sublingual tablet), is in mid-Phase 3 development for the management of fibromyalgia with a new Phase 3 study launched in the second quarter of 2022 and interim data expected in the first quarter of 2023. TNX-102 SL is also being developed to treat Long COVID, a chronic post-acute COVID-19 condition. Tonix expects to initiate a Phase 2 study in Long COVID in the third quarter of 2022. TNX-1300 (cocaine esterase) is a biologic designed to treat cocaine intoxication that is Phase 2 ready and has been granted Breakthrough Therapy Designation by the FDA. TNX-1900 (intranasal potentiated oxytocin), a small molecule in development for chronic migraine, is expected to enter the clinic with a Phase 2 study in the second half of 2022. Tonix’s rare disease portfolio includes TNX-2900 (intranasal potentiated oxytocin) for the treatment of Prader-Willi syndrome. TNX-2900 has been granted Orphan-Drug Designation by the FDA. Tonix’s immunology portfolio includes biologics to address organ transplant rejection, autoimmunity and cancer, including TNX-1500 which is a humanized monoclonal antibody targeting CD40-ligand being developed for the prevention of allograft and xenograft rejection and for the treatment of autoimmune diseases. A Phase 1 study of TNX-1500 is expected to be initiated in the second half of 2022. Tonix’s infectious disease pipeline consists of a vaccine in development to prevent monkeypox and smallpox called TNX-801, next-generation vaccines to prevent COVID-19, and a platform to make fully human monoclonal antibodies to treat COVID-19. Tonix’s lead vaccine candidates for COVID-19 are TNX-1840 and TNX-1850, which are live virus vaccines based on Tonix’s recombinant pox vector (RPV) live virus vaccine platform.
*All of Tonix’s product candidates are investigational new drugs
or biologics and none have been approved for any indication
This press release and further information about Tonix can be found at www.tonixpharma.com.
Forward
Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking words such as “anticipate,” “believe,” “forecast,” “estimate,” “expect,” and “intend,” among others. These forward-looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, risks related to the failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID-19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. Tonix does not undertake an obligation to update or revise any forward-looking statement. Investors should read the risk factors set forth in the Annual Report on Form 10-K for the year ended December 31, 2021, as filed with the Securities and Exchange Commission (the “SEC”) on March 14, 2022, and periodic reports filed with the SEC on or after the date thereof. All of Tonix’s forward-looking statements are expressly qualified by all such risk factors and other cautionary statements. The information set forth herein speaks only as of the date thereof.
Contacts
Jessica Morris (corporate)
Tonix Pharmaceuticals
investor.relations@tonixpharma.com
(862) 799-8599
Olipriya Das, Ph.D. (media)
Russo Partners
Olipriya.Das@russopartnersllc.com
(646) 942-5588
Peter Vozzo (investors)
ICR Westwicke
peter.vozzo@westwicke.com
(443) 213-0505