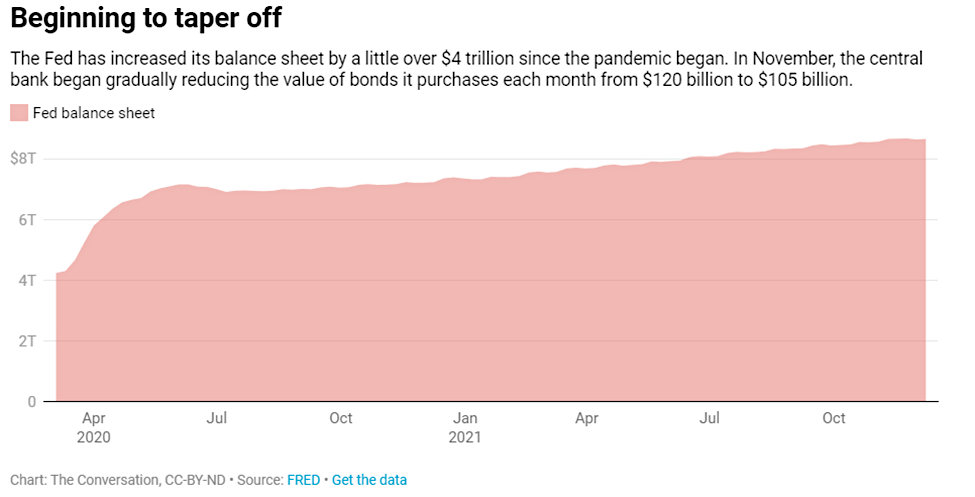
Digerati Technologies Reports 143% Revenue Growth to $3.777 Million for First Quarter FY2022
Research, News, and Market Data on Digerati Technologies
– Non-GAAP Operating EBITDA of $0.691 Million –
– Gross Profit of $2.287 Million –
– Strong Gross Margin Improvement to 60.6% –
SAN ANTONIO, TX (GlobeNewswire) – December 15, 2021 – Digerati Technologies, Inc. (OTCQB: DTGI) (“Digerati” or the “Company”), a provider of cloud services specializing in UCaaS (Unified Communications as a Service) solutions for the small to medium-sized business (“SMB”) market, announced today financial results for the three months ended October 31, 2021, the Company’s first quarter for its Fiscal Year 2022.
Key Financial Highlights for the First Quarter Fiscal Year 2022 (Ended October 31, 2021)
- Revenue increased by 143% to $3.777 million compared to $1.552 million for Q1 FY2021.
- Gross profit increased 184% to $2.287 million compared to $0.804 million for Q1 FY2021.
- Gross margin increased to 60.6% compared to 51.8% for Q1 FY2021.
- Non-GAAP Adjusted EBITDA income improved to $0.317 million, excluding all non-cash items and one-time transactional expenses, compared to Adjusted EBITDA income of $0.058 million for Q1FY2021.
- Non-GAAP operating EBITDA (OPCO EBITDA) improved to income of $0.691 million, excluding corporate expenses, compared to a non-GAAP operating EBITDA of $0.242 million for Q1 FY2021.
Arthur L. Smith, CEO of Digerati, commented, “I commend our team for continuing to execute successfully on our plan and delivering on solid financial improvements in our first quarter of FY2022. This is reflected in strong top-line revenue growth of 143%, an increase in gross margin, and improved Adjusted EBITDA results. With a solid foundation in Florida and Texas, we believe Digerati is well positioned to continue executing on its business plan and deliver on organic and acquisition growth in a very fragmented market.”
Antonio Estrada, CFO of Digerati, stated, “Our financial disciplines remain strong since acquiring Nexogy and ActivePBX in FY2021. Although most of the integration related to these acquisitions is complete, we continue to streamline cost structures and integrate systems that we anticipate will result in improved financial results in the future. We look forward to replicating this type of success with additional targeted and accretive acquisitions.”
Three Months ended October 31, 2021 Compared to Three Months ended October 31, 2020
Revenue for the three months ended October 31, 2021 was $3.777 million, an increase of $2.225 million or 143% compared to $1.552 million for the three months ended October 31, 2020. The increase in revenue between periods is primarily attributed to the consolidation of the closed acquisitions of Nexogy and ActivePBX during the period.
Gross profit for the three months ended October 31, 2021 was $2.287 million, resulting in a gross margin of 60.6%, compared to $0.804 million and 51.8% for the three months ended October 31, 2020. The increase in gross margin is primarily due to the addition of high-margin revenue associated with Nexogy’s and ActivePBX’s UCaaS product line.
Selling, General and Administrative expenses (excluding legal and professional fees) for the three months ended October 31, 2021 increased by $0.777 million, or 77%, to $1.788 million compared to $1.011 million for the three months ended October 31, 2020. The increase in SG&A is attributed to the consolidation of the closed acquisitions of Nexogy and ActivePBX.
Operating loss for the three months ended October 31, 2021, was $0.580 million, an improvement of $0.046 million or 7%, compared to $0.626 million for the three months ended October 31, 2020.
Adjusted EBITDA income for the three months ended October 31, 2021, was $0.317 million, an improvement of $0.259 million, compared to an adjusted EBITDA income of $0.058 million for the three months ended October 31, 2020. In accordance with SEC Regulation G, the non-GAAP measurement of Adjusted EBITDA has been reconciled to the nearest GAAP measurement, which can be viewed under the heading “Reconciliation of Net Loss to Adjusted EBITDA” in the financial table included in this press release.
Of note were the following non-cash expenses associated with the three months ended October 31, 2021. The Company recognized stock-based compensation and warrant expense of $0.024 million and depreciation and amortization expense of $0.492 million. Gain on derivative instruments was $4.433 million for the three months ended October 31, 2021.
Non-GAAP operating EBITDA (OPCO EBITDA) for the three months ended October 31, 2021 improved to income of $0.691 million, excluding corporate expenses, compared to a non-GAAP operating income of $0.242 million for the three months ended October 31, 2020.
Net income for the three months ended October 31, 2021, was $2.419 million, an increase of $3.145 million, as compared to a net loss of $0.726 million, for the three months ended October 31, 2020. The resulting Basic EPS for the three months ended October 31, 2021 was $0.02, as compared to a Basic EPS loss of ($0.01) for the three months ended October 31, 2020.
At October 31, 2021, Digerati had $1.646 million of cash.
Use of Non-GAAP Financial Measurements
The Company believes that EBITDA (earnings before interest, taxes, depreciation and amortization) is useful to investors because it is commonly used in the cloud communications industry to evaluate companies on the basis of operating performance and leverage. Adjusted EBITDA provides an adjusted view of EBITDA that takes into account certain significant non-recurring transactions, if any, such as impairment losses and expenses associated with pending acquisitions, which vary significantly between periods and are not recurring in nature, as well as certain recurring non-cash charges such as changes in fair value of the Company’s derivative liabilities and stock based compensation. The Company also believes that Adjusted EBITDA provides investors with a measure of the Company’s operational and financial progress that corresponds with the measurements used by management as a basis for allocating resources and making other operating decisions. Although the Company uses Adjusted EBITDA as one of several financial measures to assess its operating performance, its use is limited as it excludes certain significant operating expenses. Non-GAAP operating EBITDA (OPCO EBITDA) is useful to investors because it reflects EBITDA for the core operation of the business excluding corporate expenses, non-cash expenses and transactional expenses. EBITDA, Adjusted EBITDA, and Non-GAAP operating EBITDA are not intended to represent cash flows for the periods presented, nor have they been presented as an alternative to operating income or as an indicator of operating performance and should not be considered in isolation or as a substitute for measures of performance prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). In accordance with SEC Regulation G, the non-GAAP measurements
in this press release have been reconciled to the nearest GAAP measurement, which can be viewed under the heading “Reconciliation of Net Loss to Adjusted EBITDA” in the financial table included in this press release.
About Digerati Technologies, Inc.
Digerati Technologies, Inc. (OTCQB: DTGI) is a provider of cloud services specializing in UCaaS (Unified Communications as a Service) solutions for the business market. Through its operating subsidiaries T3 Communications (T3com.com) and Nexogy (Nexogy.com), the Company is meeting the global needs of businesses seeking simple, flexible, reliable, and cost-effective communication and network solutions including cloud PBX, cloud telephony, cloud WAN, cloud call center, cloud mobile, and the delivery of digital oxygen on its broadband network. The Company has developed a robust integration platform to fuel mergers and acquisitions in a highly fragmented market as it delivers business solutions on its carrier-grade network and Only in the Cloud™. For more information, please visit www.digerati-inc.com or follow DTGI on LinkedIn, Twitter and Facebook.
Forward-Looking Statements
The information in this news release includes certain forward-looking statements that are based upon assumptions that in the future may prove not to have been accurate and are subject to significant risks and uncertainties, including statements related to the future financial performance of the Company. Although the Company believes that the expectations reflected in the forward-looking statements such as anticipated improvement in financial results and delivering on organic and acquisition growth in a very fragmented market, are reasonable, it can give no assurance that such expectations or any of its forward-looking statements will prove to be correct. Factors that could cause results to differ include, but are not limited to, our inability to source suitable acquisition targets, failure to execute growth strategies, lack of product development and related market acceptance, the impact of competitive services and pricing, general economic conditions, and other risks and uncertainties described in the Company’s periodic filings with the Securities and Exchange Commission.
Facebook: Digerati Technologies, Inc.
Twitter: @DIGERATI_IR
LinkedIn: Digerati Technologies, Inc.
Investors
The Eversull Group
Jack Eversull
jack@theeversullgroup.com
(972) 571-1624
ClearThink
Brian Loper
bloper@clearthink.capital
(347) 413-4234