
CanAlaska Deals Three Uranium Projects in the Athabasca Basin
Terra Uranium have Staged Option to Earn up to 80% Interest in Two Properties and up to 20% Interest in One Property
Focus on High-Grade Eastern Athabasca Uranium Discovery
Vancouver, Canada, September 23, 2021 – CanAlaska Uranium Ltd. (TSX-V: CVV; OTCQB: CVVUF; Frankfurt: DH7N) (“CanAlaska” or the “Company”) is pleased to announce it has entered into a Letter of Intent (“LOI”) with Terra Uranium Pty Ltd (“Terra”), an Australian private limited corporation, to allow Terra to earn up to an 80% interest in CanAlaska’s 100%-owned Waterbury East and McTavish projects, and up to a 20% interest in CanAlaska’s 100%-owned Waterbury South project. These projects total 5,010 hectares in the Eastern Athabasca Basin in Saskatchewan, Canada (the “Projects”) (Figure 1).
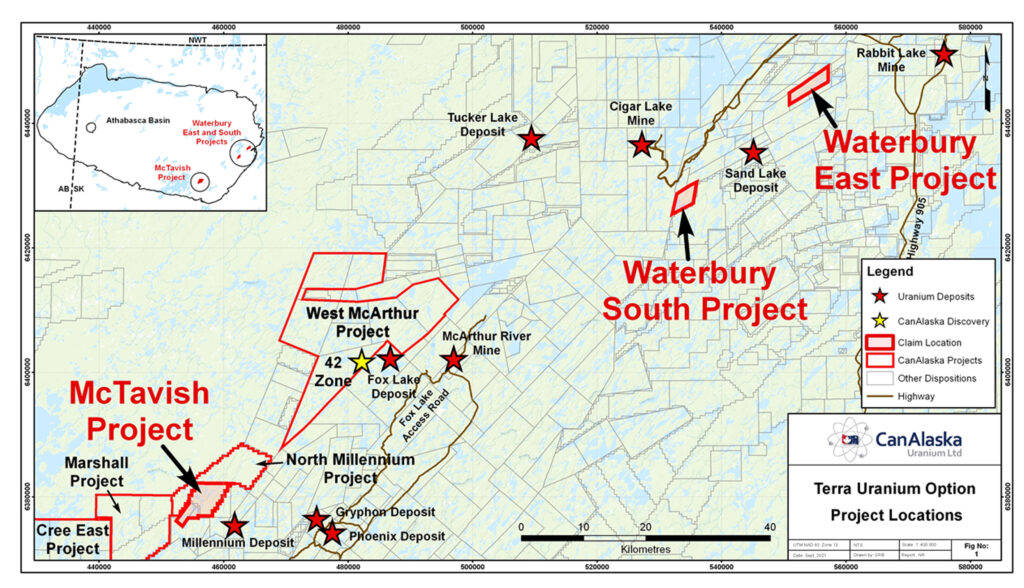
Waterbury East and McTavish Projects
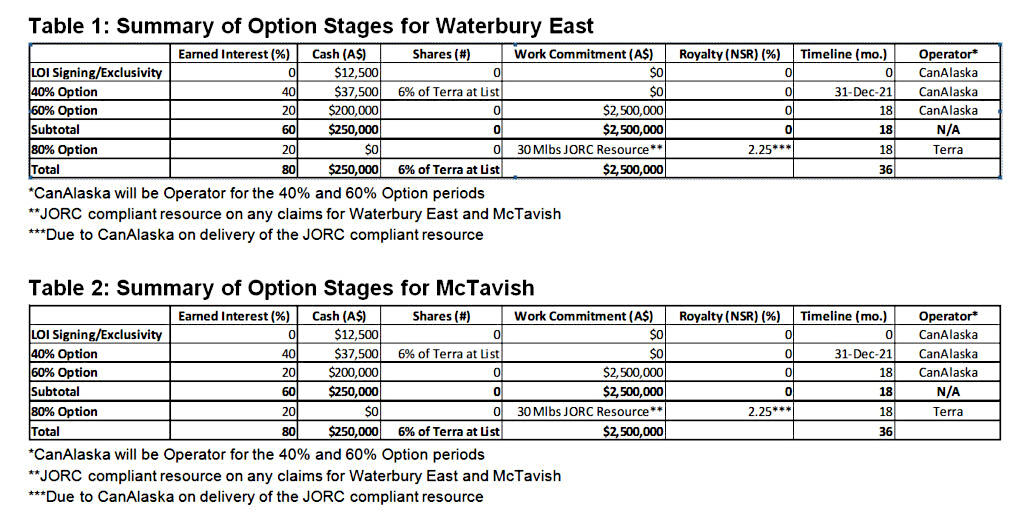
Terra may earn up to an 80% interest in each of the Waterbury East and McTavish projects by undertaking work and payments in three defined earn-in stages on each project (tables 1 and 2). Cumulatively, Terra may earn an initial 40% interest (“40% Option”) in the projects by paying the Company A$100,000 cash and issuing 12% worth of common shares at listing on the Australian Securities Exchange (“ASX”) by December 31, 2021. Cumulatively, Terra may earn an additional 20% interest (“60% Option”) in the projects by paying a further A$400,000 and incur A$5,000,000 in exploration expenditures within 18 months of ASX approval date. Cumulatively, Terra may earn an additional 20% interest (“80% Option”) in the projects by delivering and filing a JORC compliant resource of at least 30,000,000 pounds U3O8 on any of the Waterbury East or McTavish claims, and granting to the Company a 2.25% net smelter returns (NSR) royalty on all products derived from the claims, within 36 months of ASX listing date. CanAlaska will be operator of the projects through the 60% Option threshold and charge a 20% operator fee to Terra.
After successful completion of either of the 40% Option or 60% Option stages of the agreement, and if Terra elects to not enter the final stage, a joint venture will be formed and the parties will co-contribute on a simple pro-rata basis or dilute on a pre-defined straight-line dilution formula. If either party dilutes to a 10% interest, the diluting party will automatically forfeit its interest in the respective project and in lieu thereof will be granted a 2.0% net smelter returns (NSR) royalty on the respective property.
An area of mutual interest will be established that extends two kilometres from the boundary of the claims.
Waterbury South Project
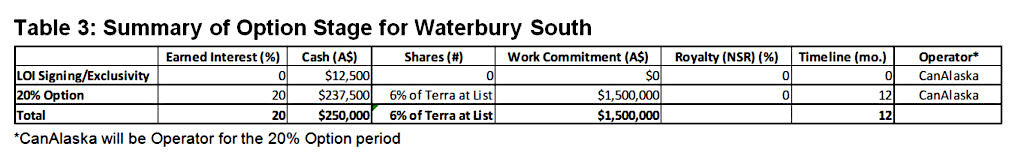
Terra may earn up to a 20% interest in the Waterbury South Project by undertaking work and payments in one defined earn-in stage (Table 3). Terra may earn the 20% interest (“20% Option”) by paying the Company A$250,000 cash, issuing 6% worth of common shares at listing on the ASX (listing due by December 31, 2021), and incurring A$1,500,000 in exploration expenditures within 12 months of ASX listing date. CanAlaska will be operator of the project through the 20% Option and charge a 20% operator fee to Terra.
After successful completion of the 20% Option stage of the agreement, a joint venture will be formed and the parties will co-contribute on a simple pro-rata basis or dilute on a pre-defined straight-line dilution formula.
An area of mutual interest will be established that extends two kilometres from the boundary of the claims.
About Terra Uranium Pty Ltd
Terra Uranium Pty Ltd is an Australian private limited corporation that is in the process of undergoing an initial public offering and concurrent listing on the Australian Securities Exchange (ASX). It is a condition of this transaction that Terra be listed on the ASX.
CanAlaska CEO, Cory Belyk, comments, “CanAlaska is pleased to work with Terra Uranium, a pending new Australian-listed player in the Basin, to help fund the next stage of exploration on these highly prospective Eastern Athabasca uranium projects. This significant investment by Terra will allow CanAlaska to achieve its objective of being a hybrid explorer and project generator by moving these projects toward discovery and preserving up-side without diluting current shareholders.”
Other News
The Company is currently drilling on its West McArthur Joint Venture Project in the 42 Zone discovery area, a joint venture with Cameco Corportation. Denison Mines is currently drilling on the Company’s new Moon Lake South Joint Venture near Denison’s Wheeler River Project.
About CanAlaska Uranium
CanAlaska Uranium Ltd. (TSX-V: CVV; OTCQB: CVVUF; Frankfurt: DH7N) holds interests in approximately 214,000 hectares (530,000 acres), in Canada’s Athabasca Basin – the “Saudi Arabia of Uranium.” CanAlaska’s strategic holdings have attracted major international mining companies. CanAlaska is currently working with Cameco and Denison at two of the Company’s properties in the Eastern Athabasca Basin. CanAlaska is a project generator positioned for discovery success in the world’s richest uranium district. The Company also holds properties prospective for nickel, copper, gold and diamonds.
The qualified technical person for this news release is Nathan Bridge, MSc., P.Geo., CanAlaska’s Vice President, Exploration.
For further information visit www.canalaska.com.
On behalf of the Board of Directors
“Peter Dasler”
Peter Dasler, M.Sc., P.Geo.
President
CanAlaska Uranium Ltd.
Contacts:
Peter Dasler, President
Tel: +1.604.688.3211 x 138
Email: info@canalaska.com
Cory Belyk, CEO and Executive Vice President
Tel: +1.604.688.3211 x 138
Email: cbelyk@canalaska.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward-looking information
All statements included in this press release that address activities, events or developments that the Company expects, believes or anticipates will or may occur in the future are forward-looking statements. These forward-looking statements involve numerous assumptions made by the Company based on its experience, perception of historical trends, current conditions, expected future developments and other factors it believes are appropriate in the circumstances. In addition, these statements involve substantial known and unknown risks and uncertainties that contribute to the possibility that the predictions, forecasts, projections and other forward-looking statements will prove inaccurate, certain of which are beyond the Company’s control. Readers should not place undue reliance on forward-looking statements. Except as required by law, the Company does not intend to revise or update these forward-looking statements after the date hereof or revise them to reflect the occurrence of future unanticipated events.



























