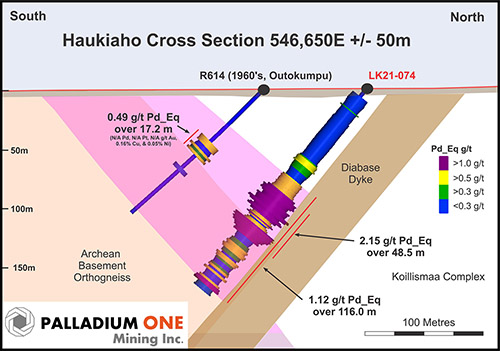
Lineage Cell Therapeutics Reports Additional Cases Of Retinal Tissue Restoration In Dry AMD Patients Treated With Opregen RPE Cells
- Restoration Was Observed in Two Additional Patients in a Phase 1/2a Clinical Study of OpRegen
- An Earlier and First-Known Clinical Report of Restoration Has Been Maintained for 3 Years
- Restoration Has Been Observed in Three of Four Patients Who Received OpRegen RPE Cells Across a Wide Area of Atrophy
CARLSBAD, Calif.–(BUSINESS WIRE)–Jun. 1, 2021–
Lineage Cell Therapeutics, Inc.
(NYSE American and TASE: LCTX), a clinical-stage biotechnology company developing allogeneic cell therapies for unmet medical needs, announced today that restoration of retinal tissue was observed in two additional patients enrolled in the Company’s Phase 1/2a study of its lead product candidate, OpRegen. OpRegen is an allogeneic retinal pigment epithelium (RPE) cell transplant therapy in development for the treatment of age-related macular degeneration (AMD) with geographic atrophy (GA), or dry (atrophic) AMD. These new findings occurred in two of the three Cohort 4 patients treated in November of 2020, where surgeons successfully covered the majority of the area of atrophy with a suspension of OpRegen cells. Outer retinal layer restoration, which was observed using clinical high-resolution Optical Coherence Tomography (OCT), was evidenced by the presence of new areas of RPE monolayer with overlying ellipsoid zone, external limiting membrane, and outer nuclear layer, which were not present at the time of baseline assessment. These findings suggest integration of the new RPE cells with functional photoreceptors in areas that previously showed no presence of any of these cells. These effects were most prominent in the transitional areas around the primary area of GA. In addition to positive anatomical changes, all three patients’ visual acuity increased above baseline levels within 6 months post-transplant. The totality of these findings supports the view that atrophic AMD is not an irreversible, degenerative condition and that some portion of diseased retinal tissue may be recoverable.
In connection with these findings, Lineage has filed a Patent Cooperation Treaty (PCT) patent application which describes restoration of the anatomy and/or functionality of diseased retinas. The application is based on clinical trial data demonstrating, for the first time, administration of RPE cells to an atrophic retina restores structure and function to one or more retinal layers. Allowed claims of a national patent application based on the PCT application would have an estimated expiration date of at least
May 24, 2041.
“After reporting the first case of retinal restoration last year, it is extremely exciting for me to see retinal restoration replicated in additional OpRegen-treated patients. Confirmation of restoration was performed by independent experts using multimodality imaging techniques and these new cases reinforce earlier findings that treatment with OpRegen can save dysfunctional photoreceptors and replace RPE cells in areas of geographic atrophy, which could provide a remarkable treatment opportunity for this patient population,” stated Jordi Monés, M.D., Ph.D., Director, Institut de la Màcula and Director, Principal Investigator and Founder,
Barcelona Macula Foundation. “It has long been hypothesized that in atrophic AMD patients, cells in the transition areas at the boundaries of the GA are dysfunctional and dying, but not completely lost. These unprecedented findings provide further evidence that the addition of new RPE cells may restore the microenvironment of the surrounding tissue and contribute to the survival and function of existing cells that otherwise, if left untreated, would inevitably progress to further expansion of the atrophic region.”
“To our knowledge, these three patients represent the only examples of an experimental treatment for dry AMD demonstrating a reduction, rather than attenuating further expansion, of an area of atrophy in humans,” stated
Brian M. Culley, Lineage CEO. “Now that these findings of retinal restoration have been confirmed in multiple patients over clinically meaningful time periods, we believe we are in a position to pioneer a new paradigm for how we and others evaluate and treat atrophic AMD. Notably, 75% of patients who received broad coverage of OpRegen across the area of GA exhibited evidence of restoration, indicating that clinical benefits may be improved by a more central and complete placement of OpRegen cells across the atrophic area. Importantly, in addition to thickening of the outer nuclear layers and restoration of retinal structures in these patients, we also have observed durable improvements in visual acuity. Additional evidence collected recently supports our belief that treatment with OpRegen can provide patients not only with improvements in the anatomy and the structure of their retina and visual acuity, but also enhance quality of life. We will discuss these new findings with our advisors in the coming weeks and months to assess implications for the clinical development and regulatory approval pathways for OpRegen. We believe these findings represent a significant advancement in the field of cell therapy, in which the directed differentiation and transplant of specific cell types to treat severe diseases and conditions may generate outcomes which have remained out of the reach of traditional molecular approaches.”
The loss of RPE cells over time creates progressively larger areas of atrophy in the adult retina, leading to impaired vision or complete blindness, a condition known as atrophic AMD. Humans lack the innate ability to regenerate retinal tissue and replace lost retina cells, which led to a presumption that progression of GA may someday be slowed or halted but could not be reversed. The unique findings from the ongoing OpRegen clinical study support a different view, in which an RPE cell transplant can potentially replace or rescue retinal cells in patients who suffer from retinal lesions or degeneration. Notably, in the two additional Cohort 4 patients evidencing retinal restoration, in peripheral areas of incomplete RPE and outer retinal atrophy (iRORA), away from the primary atrophic lesion, there were examples of complete resolution following OpRegen transplant in some of the iRORA lesions. Additionally, in some areas of complete RPE and outer retinal atrophy (cRORA), similar, full-thickness restoration of retinal layers was observed, particularly in areas of the outer periphery of the transition zone. The transition zone may represent the ideal area to target with cell therapy as the surviving photoreceptors may be restored to normal function with the transplant of new, allogeneic RPE. The first Cohort 4 patient with evidence of retinal restoration and confirmed history of GA growth, first reported last year, continues to demonstrate areas of retinal restoration through that patient’s most recent clinical visit, which occurred approximately 3 years post-transplant. These new findings have been confirmed utilizing multiple imaging technologies, including OCT, a non-invasive test which uses light waves to take cross-sectional images of the retina, allowing for visualization of each of the distinctive layers. The use of multiple imaging modalities differs from traditional assessment of GA progression, which employs only fundus autofluorescence (FAF) to assess changes in the total surface area of the apparent GA over time. Using only FAF may fail to identify structural changes that can be observed only with the addition of OCT imaging. The use of OCT allows for a more precise determination of changes in retinal thickness, organization, and overall health of the retina in areas of potential atrophy, which are possible with cell therapy. These changes may be more likely to be associated with improvements in visual acuity, reading speed, and quality of life over time.
Overall, OpRegen has been well tolerated with no unexpected adverse events or serious adverse events, and evidence of durable engraftment of OpRegen RPE cells have extended to more than 5 years post-transplant in earliest treated patients.
Dr. Monés has served as an external expert consultant for the OpRegen program for approximately 5 years. Dr. Monés completed his retinal specialist training at the
Massachusetts Eye and Ear Infirmary at
Harvard University, and earned his Ph.D. degree in Medicine and Surgery at the
University of Barcelona.
Lineage plans to host a webinar with external therapeutic area experts to discuss the findings in detail, including a review of anatomical improvements, functional activity, and additional results of treatment with OpRegen. The Company will announce the date and time for this event in the coming days. The webinar will be available in the Events and Presentations section of Lineage’s website.
About OpRegen
OpRegen is currently being evaluated in a Phase 1/2a open-label, dose escalation safety and efficacy study of a single injection of human retinal pigment epithelium cells derived from an established pluripotent cell line and transplanted subretinally in patients with advanced dry AMD with GA. The study enrolled 24 patients into 4 cohorts. The first 3 cohorts enrolled only legally blind patients with Best Corrected Visual Acuity (BCVA) of 20/200 or worse. The fourth cohort enrolled 12 better vision patients (BCVA from 20/65 to 20/250 with smaller mean areas of GA). Cohort 4 also included patients treated with a new “thaw-and-inject” formulation of OpRegen, which can be shipped directly to sites and used immediately upon thawing, removing the complications and logistics of having to use a dose preparation facility. The primary objective of the study is to evaluate the safety and tolerability of OpRegen as assessed by the incidence and frequency of treatment emergent adverse events. Secondary objectives are to evaluate the preliminary efficacy of OpRegen treatment by assessing the changes in ophthalmological parameters measured by various methods of primary clinical relevance. OpRegen is a registered trademark of
Cell Cure Neurosciences Ltd., a majority-owned subsidiary of
Lineage Cell Therapeutics, Inc.
About Age-Related Macular Degeneration
Age-related macular degeneration (AMD) is an eye disease that can blur the sharp, central vision in patients and is the leading cause of vision loss in people over the age of 60. There are two forms of AMD: dry (atrophic) AMD and wet (neovascular) AMD. Dry (atrophic) AMD is the more common of the two forms, accounting for approximately 85-90% of all cases. In atrophic AMD, parts of the macula get thinner with age and accumulations of extracellular material between Bruch’s membrane and the RPE, known as drusen, increase in number and volume, leading to a progressive loss of central vision, typically in both eyes. Global sales of the two leading wet AMD therapies were in excess of
$10 billion in 2019. Nearly all cases of wet AMD eventually will develop the underlying atrophic AMD if the newly formed blood vessels are treated correctly. There are currently no
U.S. Food and Drug Administration, or
European Medicines Agency, approved treatment options available for patients with atrophic AMD.
About Lineage Cell Therapeutics, Inc.
Lineage Cell Therapeutics is a clinical-stage biotechnology company developing novel cell therapies for unmet medical needs. Lineage’s programs are based on its robust proprietary cell-based therapy platform and associated in-house development and manufacturing capabilities. With this platform Lineage develops and manufactures specialized, terminally differentiated human cells from its pluripotent and progenitor cell starting materials. These differentiated cells are developed to either replace or support cells that are dysfunctional or absent due to degenerative disease or traumatic injury or administered as a means of helping the body mount an effective immune response to cancer. Lineage’s clinical programs are in markets with billion dollar opportunities and include three allogeneic (“off-the-shelf”) product candidates: (i) OpRegen®, a retinal pigment epithelium transplant therapy in Phase 1/2a development for the treatment of dry age-related macular degeneration, a leading cause of blindness in the developed world; (ii) OPC1, an oligodendrocyte progenitor cell therapy in Phase 1/2a development for the treatment of acute spinal cord injuries; and (iii) VAC2, an allogeneic dendritic cell therapy produced from Lineage’s VAC technology platform for immuno-oncology and infectious disease, currently in Phase 1 clinical development for the treatment of non-small cell lung cancer. For more information, please visit www.lineagecell.com or follow the Company on Twitter @LineageCell.
Forward-Looking Statements
Lineage cautions you that all statements, other than statements of historical facts, contained in this press release, are forward-looking statements. Forward-looking statements, in some cases, can be identified by terms such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “design,” “intend,” “expect,” “could,” “can,” “plan,” “potential,” “predict,” “seek,” “should,” “would,” “contemplate,” project,” “target,” “tend to,” or the negative version of these words and similar expressions. Such statements include, but are not limited to, statements relating to the potential benefits of treatment with OpRegen in dry AMD patients with GA, the significance of clinical data reported to date, including the findings of retinal restoration, from the ongoing Phase 1/2a of OpRegen, and the potential for issuance of new patents relating to OpRegen. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause Lineage’s actual results, performance or achievements to be materially different from future results, performance or achievements expressed or implied by the forward-looking statements in this press release, including risks and uncertainties inherent in Lineage’s business and other risks in Lineage’s filings with the
Securities and Exchange Commission (SEC). Lineage’s forward-looking statements are based upon its current expectations and involve assumptions that may never materialize or may prove to be incorrect. All forward-looking statements are expressly qualified in their entirety by these cautionary statements. Further information regarding these and other risks is included under the heading “Risk Factors” in Lineage’s periodic reports with the
SEC, including Lineage’s most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q filed with the
SEC and its other reports, which are available from the SEC’s website. You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date on which they were made. Lineage undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
Lineage Cell Therapeutics, Inc. IR
Ioana C. Hone
(ir@lineagecell.com)
(442) 287-8963
Solebury Trout IR
Gitanjali Jain Ogawa
(Gogawa@soleburytrout.com)
(646) 378-2949
Russo Partners – Media Relations
Nic Johnson or
David Schull
Nic.johnson@russopartnersllc.com
David.schull@russopartnersllc.com
(212) 845-4242
Source:
Lineage Cell Therapeutics, Inc.