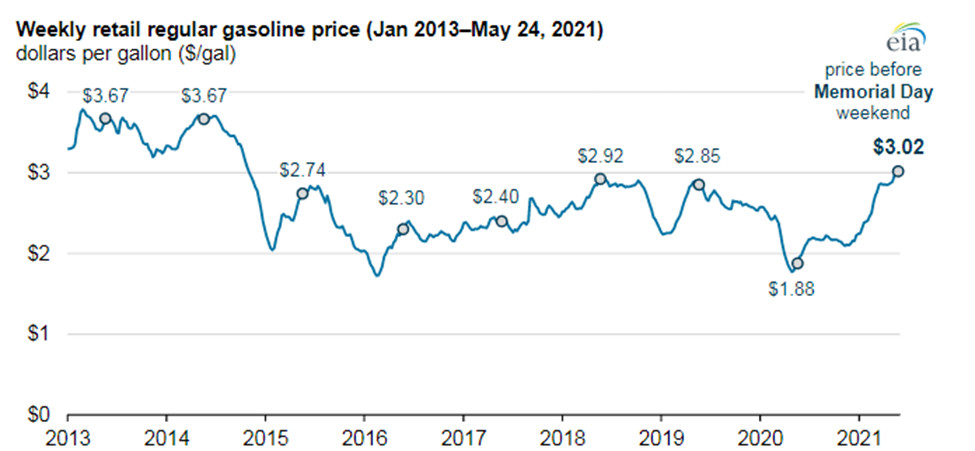
Stem Cell Science and The New Age of Therapeutic Discovery
Recent advances in stem cell technologies, armed with the ability to generate almost any tissue-specific cell types of the body in a dish, human pluripotent stem cells (hPSCs) have drastically moved therapeutic research forward in unprecedented ways. Stem cell-derived cells are increasingly recognized for their potential in cell replacement therapy – that is the replacement of dying or damaged tissues in the treatment of chronic diseases and injuries, but stem cells are also playing important roles in driving drug discovery, screening, research, and development efficiently and cost-effectively [1].
Advantages Over Traditional Methods
Traditional methods of drug development involve pre-clinical trials on animals, primary cells, or even transformed cell lines. However, there are limitations to such methods. Firstly, animal physiology differs from that of humans, therefore disease manifestations [2], as well as responses towards compounds and drugs, may vary from humans [3]. Results from animal studies rarely correlate with human clinical trials [4]. In addition, animal research is very costly and highly variable [5]. Next, primary cells (either isolated from living or cadaveric donors) are finite in numbers; only a limited number of tests can be performed on these cells. Lastly, transformed cell lines are genetically manipulated so that they remain proliferative in order to obtain large quantities of material for drug screening and testing. However, these cells do not accurately represent native cells present in patients.
Because hPSCs are highly proliferative and capable of self-renewal, they can be easily expanded, providing an essentially unlimited source of cells. At present, it is estimated that we can generate more than 200 cell types from hPSCs [5]. This translates into the ability to produce large quantities of any cell type from hPSCs for therapeutic research. Not only does this mean that studies can be performed on various types of cells for various diseases, testing efforts can also be scaled up and accelerated while remaining to be affordable and sustainable. In vitro toxicity tests can also be easily conducted in tandem [6].

Generating ‘Diseased’ Stem Cells
Before we can begin screening and test therapeutics, we need to produce cells or tissues from diseased hPSCs. There are a few ways this can be achieved. Firstly, the discovery of induced pluripotent stem cells (iPSCs) by Nobel Prize winner Shinya Yamanaka and colleagues in 2017 [7,8] has allowed us to generate patient-specific stem cells from any patients’ cells [9] (from blood, skin biopsies, urine, etc) by simply introducing four key transcription factors into the cells. This process is termed cellular reprogramming. Despite undergoing this process of cellular reprogramming, iPSCs and their associated iPSC-derived cells, are able to preserve the original genetic mutations (if any) and disease phenotypes, therefore faithfully mimicking human disease in vitro. Over the past decade, disease-specific iPSCs have been successful in modeling a wide range of complex human diseases [1], and are therefore poise as an attractive cellular model for pharmacological testing.
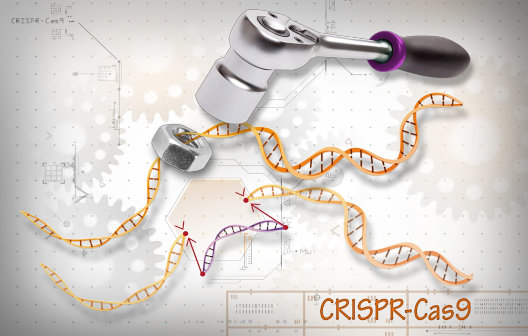
Secondly, the discovery of the CRISPR-Cas9 technology [10] has revolutionized genetic editing. CRISPR-Cas9 editing system can be employed to induce genetic mutations into either healthy human embryonic stem cells or iPSCs that are known to cause certain diseases, thereby creating ‘diseased’ stem cells. These genetically edited stem cells can then be propagated and differentiated into ‘diseased’ cells (e.g. cardiomyocytes, neurons, etc.) for therapeutic discovery and screening [11].
Success Stories
In 2018, Tabata et al. [12] differentiated Parkinson disease (PD) patient-iPSCs (harboring PARK2 mutations; familial PD) into dopaminergic neurons and screened the diseased neurons with an FDA-approved drug library. They discovered that a drug, a calcium channel antagonist, was able to prevent neuronal death associated with the disease.
Interestingly, researchers have recently devised a co-culture system comprising of hPSC-derived endothelial cells and hepatocytes (liver cells) to test nutraceuticals. This endothelial-hepatic platform allows for the systemic effects of drug metabolism to be included in the study, which will otherwise be excluded if only endothelial cells were tested on [13].
Most recently, a group of researchers utilized iPSCs of patients with cardiac rhythm disorder long QT syndrome 3 (LQT3) and differentiated the cells into cardiomyocytes to perform HTS for anti-arrhythmic drugs. Using the LQT3-cardiomyocytes, they were able to improve their drug design and successfully tested their drug on the diseased cardiomyocytes of diverse backgrounds [14].
Moving Forward
hPSCs and the advancements of stem cell research have given us the capacity to generate large volumes of healthy and diseased tissue-specific cells that are true to human physiology. This has opened doors to large-scale, high-throughput drug discovery and screening, facilitating pre-clinical drug development efforts. Unlike traditional methods, hPSC-based drug development platforms are more scalable, accurate, and cost-effective.
As the field of stem cell research progresses, we can look forward to improved iPSC reprogramming protocols that increase the efficiency of obtaining patient-specific iPSCs, as well as differentiation protocols that improve the quality of differentiated cells. Efforts are also currently channeled towards devising methods to better grow and maintain stem cell-derived cells in culture. This will permit a longer duration of therapeutic and toxicity testing without cells losing their cellular identity or molecular signatures. To address concerns related to the discrepancy between the effects of drugs in vitro and in vivo, researchers have also recently explored the use of hPSC-derived organoids to model human diseases, and then perform therapeutic testing on these three-dimensional ‘mini organs’ [15]. hPSC-derived organoids are composed of multiple cell types and can better recapitulate tissue physiology and hence, clinical features of diseases.
Therefore, stem cells have opened doors to a new age of therapeutic discovery and development that promises more treatment options in the coming years, even for rare and complex human diseases.
About the Author:
Nicole Pek is a stem cell biologist and enthusiastic science
communicator. She has worked on using human pluripotent stem cells to study
cellular development in multiple organ systems, to model complex human
diseases, and screen for therapeutics that could treat the diseases. Outside of
the lab, Nicole plays a pro-active role in communicating to the public through
her science blog ‘Two Cells’ and her education podcast ‘The Diploid Duo’.
Suggested Reading:
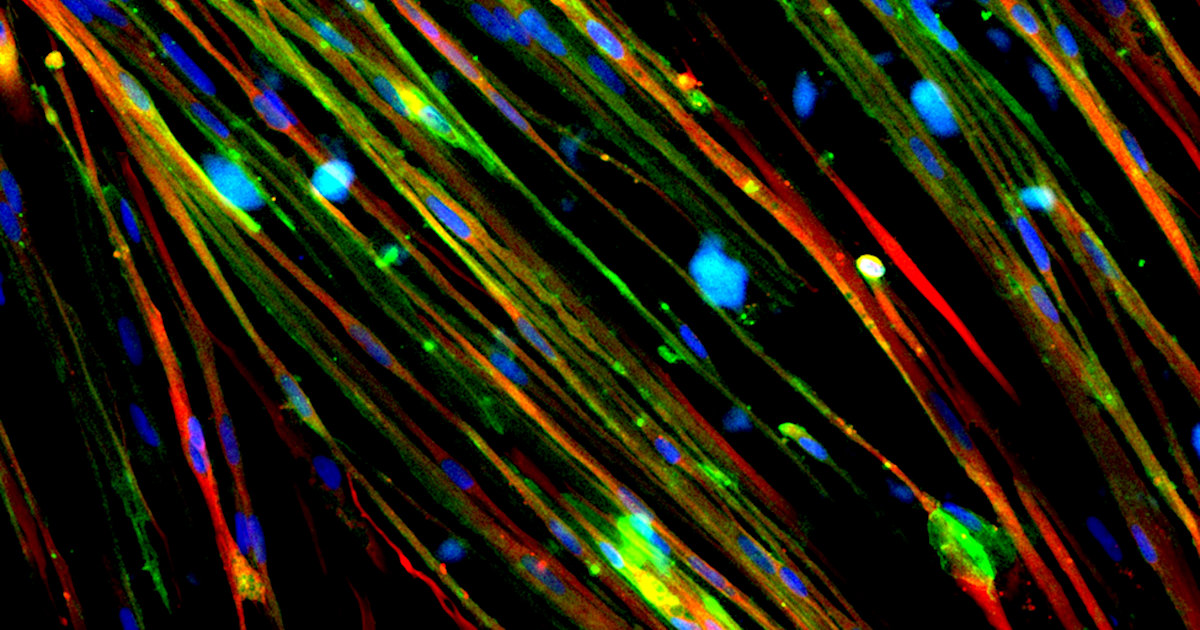
About the Image:
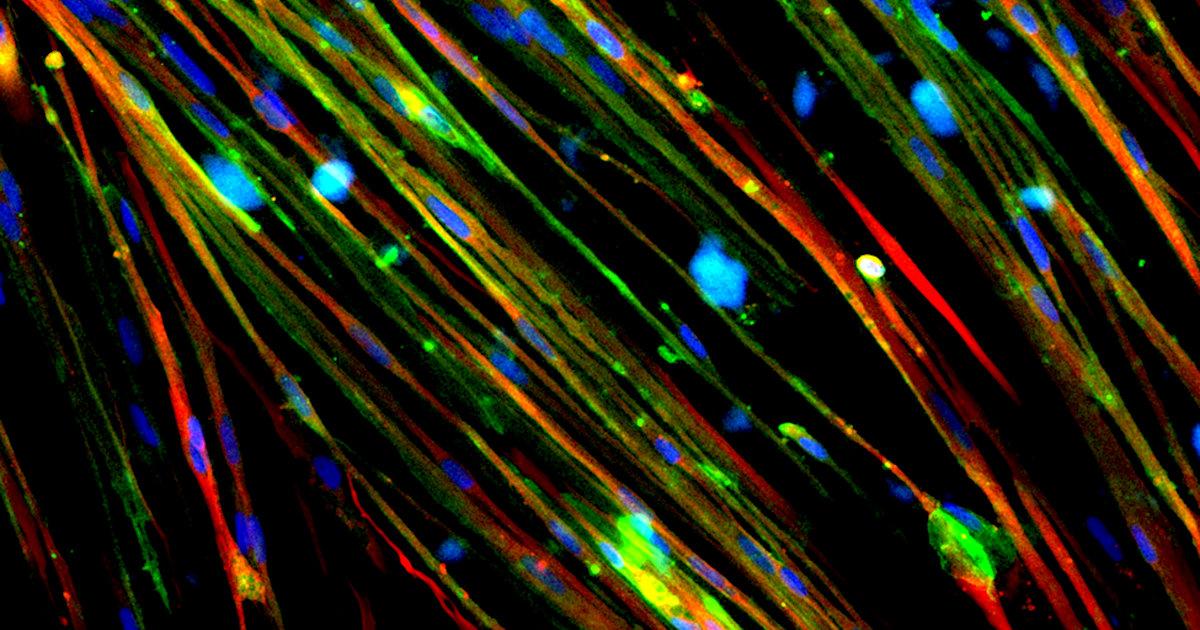
Restoration of Dystrophin in Duchenne Muscular Dystrophy Cells with Gene Editing
This image shows the restoration of
dystrophin (stained green) in Duchenne muscular dystrophy (DMD) muscle cells derived
from human induced pluripotent stem cells. DMD is caused by mutations in the
DMD gene that affect the production of dystrophin, a protein involved in muscle
cell membrane structure. Researchers used CRISPR/Cas9 gene editing technology
to correct a mutation, resulting in dystrophin restoration. This technology
could be therapeutic in up to 60% of DMD patient mutations. Nuclei in the
muscle cells are stained blue and the contractile protein myosin is stained
red.
Credit: Courtney Young, M.S., Melissa Spencer lab, University of California, Los Angeles.
References
1. Inoue H, Yamanaka S. The Use of Induced Pluripotent Stem Cells in Drug Development. Clin Pharmacol Ther 2011;89:655–61.
2. Justice MJ, Dhillon P. Using the mouse to model human disease: increasing validity and reproducibility. Dis Model
Mech 2016;9:101–3.
3. Zimmerman S. Why Drugs Tested in Mice Fail in Human Clinical Trials. Sci News 2020.
4. Van Norman GA. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl Sci 2019;4:845–54.
5. Boheler KR. Stem Cell Pluripotency: A Cellular Trait that Depends on Transcription Factors, Chromatin State and a Checkpoint Deficient Cell Cycle. J Cell Physiol 2009;221:10–7.
6. Hook LA. Stem cell technology for drug discovery and development. Drug Discov Today 2012;17:336–42.
7. Takahashi K, Tanabe K, Ohnuki M et
al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–72.
8. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–76.
9. Liu G, David BT, Trawczynski M et
al. Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Rev Rep 2020;16:3–32.
10. Ran FA, Hsu PD, Wright J et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 2013;8:2281–308.
11. Patmanathan SN, Gnanasegaran N, Lim MN et al. CRISPR/Cas9 in Stem Cell Research: Current Application and Future Perspective. Curr Stem Cell Res Ther 2018;13:632–44.
12. Tabata Y, Imaizumi Y, Sugawara M et
al. T-type Calcium Channels Determine the Vulnerability of Dopaminergic Neurons to Mitochondrial Stress in Familial Parkinson Disease. Stem Cell Rep 2018;
11:1171–84.
13. Narmada BC, Goh YT, Li H et al. Human Stem Cell?Derived Endothelial?Hepatic Platform for Efficacy Testing of Vascular?Protective Metabolites from Nutraceuticals. Stem Cells Transl Med 2017;
6:851–63.
14. McKeithan WL, Feyen DAM, Bruyneel AAN
et al. Reengineering an Antiarrhythmic Drug Using Patient hiPSC Cardiomyocytes to Improve Therapeutic Potential and Reduce Toxicity. Cell
Stem Cell 2020;27:813-821.e6.
15. Ho BX, Pek NMQ, Soh B-S. Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells. Int J Mol Sci 2018;19, DOI: 10.3390/ijms19040936.

Stay up to date. Follow us:
Stay up to date. Follow us: