
Sierra Metals Announces Results Of Annual General Meeting Of Shareholders
TORONTO–(BUSINESS WIRE)– Sierra Metals Inc. (TSX: SMT) (BVL: SMT) (NYSE AMERICAN: SMTS) (“Sierra Metals” or the “Company”) hereby announces the voting results from the Company’s Annual General Meeting of Shareholders held on Thursday, June 10, 2021 (the “Meeting”).
A total of 133,132,611 common shares were voted at the Meeting, being 81.46% of the Company’s issued and outstanding common shares. Shareholders voted in favour of all matters brought before the Meeting, including the re-appointment of PricewaterhouseCoopers LLP as the Company’s auditors for the ensuing year, and the election of management’s nominees to the Company’s board of directors (the “Board”).
Detailed results of the votes on the election of directors are as follows:
|
Director |
Votes For |
Votes Withheld |
Outcome of |
|
Jose Vizquerra |
78,891,458 (62.64%) |
47,048,136 (37.36%) |
Approved |
|
J. Alberto Arias |
58,459,843 (46.42%) |
67,479,751 (53.58%) |
Approved |
|
Ricardo Arrarte |
72,338,338 (57.44%) |
53,601,256 (42.56%) |
Approved |
|
Douglas Cater |
79,034,768 (62.76%) |
46,904,826 (37.24%) |
Approved |
|
Steven Dean |
77,094,708 (61.22%) |
48,844,886 (38.78%) |
Approved |
|
Luis Marchese |
72,978,835 (57.95%) |
52,960,759 (42.05%) |
Approved |
|
Dionisio Romero |
70,474,604 (55.96%) |
55,464,990 (44.04%) |
Approved |
|
Koko Yamamoto |
71,905,376 (57.10%) |
54,034,218 (42.90%) |
Approved |
One of the eight directors elected at the Meeting, J. Alberto Arias, received a greater number of votes “withheld” from his election as a director than votes “for” his election. The results of this outcome have been defined within the Majority Voting Policy (as disclosed on the Company website – https://www.sierrametals.com/about-sierra/corporate-governance/default.aspx). The Board’s decision will be disclosed by press release.
About Sierra Metals
Sierra Metals is a diversified Canadian mining company focused on the production and development of precious and base metals from its polymetallic Yauricocha Mine in Peru, and Bolivar and Cusi Mines in Mexico. The Company is focused on increasing production volume and growing mineral resources. Sierra Metals has recently had several new key discoveries and still has many more exciting brownfield exploration opportunities at all three Mines in Peru and Mexico that are within close proximity to the existing mines. Additionally, the Company also has large land packages at all three mines with several prospective regional targets providing longer-term exploration upside and mineral resource growth potential.
The Company’s common shares trade on the Toronto Stock Exchange and the Bolsa de Valores de Lima under the symbol “SMT” and on the NYSE American Exchange under the symbol “SMTS”.
For further information regarding Sierra Metals, please visit www.sierrametals.com.
Continue to Follow, Like and Watch our progress:
Web: www.sierrametals.com | Twitter: sierrametals | Facebook: SierraMetalsInc | LinkedIn: Sierra Metals Inc | Instagram: sierrametals
Forward-Looking Statements
This press release contains “forward-looking information” and “forward-looking statements” within the meaning of Canadian and U.S. securities laws related to the Company (collectively, “forward-looking information”). Forward-looking information includes, but is not limited to, statements with respect to the Company’s operations, including anticipated developments in the Company’s operations in future periods, the Company’s planned exploration activities, the adequacy of the Company’s financial resources, and other events or conditions that may occur in the future. Statements concerning mineral reserve and resource estimates may also be considered to constitute forward-looking statements to the extent that they involve estimates of the mineralization that will be encountered if and when the properties are developed or further developed. These statements relate to analyses and other information that are based on forecasts of future results, estimates of amounts not yet determinable and assumptions of management. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, assumptions or future events or performance (often, but not always, using words or phrases such as “expects”, “anticipates”, “plans”, “projects”, “estimates”, “assumes”, “intends”, “strategy”, “goals”, “objectives”, “potential” or variations thereof, or stating that certain actions, events or results “may”, “could”, “would”, “might” or “will” be taken, occur or be achieved, or the negative of any of these terms and similar expressions) are not statements of historical fact and may be forward-looking information.
Forward-looking information is subject to a variety of risks and uncertainties, which could cause actual events or results to differ from those reflected in the forward-looking information, including, without limitation, the risks described under the heading “Risk Factors” in our Annual Information Form dated March 30, 2021 in respect of the year ended December 31, 2020 and other risks identified in the Company’s filings with Canadian securities regulators and the U.S. Securities and Exchange Commission, which filings are available at www.sedar.com and www.sec.gov, respectively.
The risk factors referred to above is not exhaustive of the factors that may affect any of the Company’s forward-looking information. Forward looking information includes statements about the future and are inherently uncertain, and the Company’s actual achievements or other future events or conditions may differ materially from those reflected in the forward-looking information due to a variety of risks, uncertainties and other factors. The Company’s statements containing forward-looking information are based on the beliefs, expectations and opinions of management on the date the statements are made, and the Company does not assume any obligation to update forward-looking information if circumstances or management’s beliefs, expectations or opinions should change, other than as required by applicable law. For the reasons set forth above, one should not place undue reliance on forward-looking information.
Mike McAllister
V.P., Investor Relations
Sierra Metals Inc.
+1 (416) 366-7777
Email: info@sierrametals.com
Ed Guimaraes
CFO
Sierra Metals Inc.
+1(416) 366-7777
Luis Marchese
CEO
Sierra Metals Inc.
+1(416) 366-7777
Source: Sierra Metals Inc.

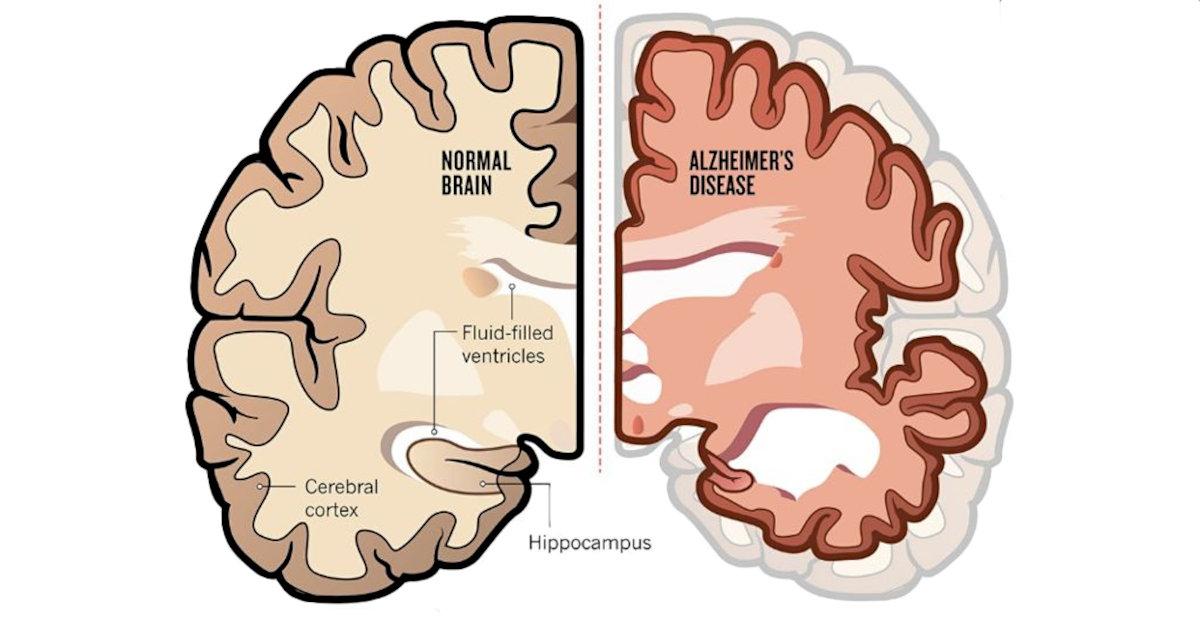
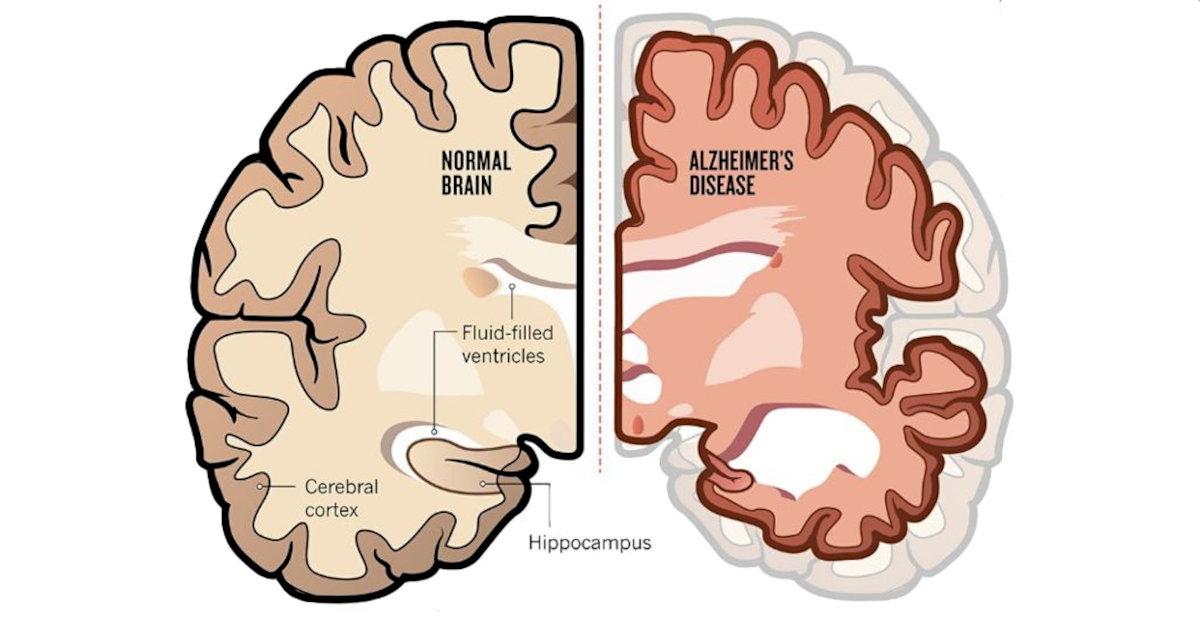




































 Michael Shine is a pharmaceutical and biotechnology executive with nearly 35 years of industry experience. Over the course of his career, Mr. Shine has held leadership positions within large pharmaceutical companies, including Novapharm Therapeutics, Colgate Oral Pharmaceutical, and Pfizer Vaccines (formerly Wyeth). He also served as Chief Marketing Officer with Thomas Reuters and spent more than eight years in the start-up pharmaceutical space.
Michael Shine is a pharmaceutical and biotechnology executive with nearly 35 years of industry experience. Over the course of his career, Mr. Shine has held leadership positions within large pharmaceutical companies, including Novapharm Therapeutics, Colgate Oral Pharmaceutical, and Pfizer Vaccines (formerly Wyeth). He also served as Chief Marketing Officer with Thomas Reuters and spent more than eight years in the start-up pharmaceutical space.