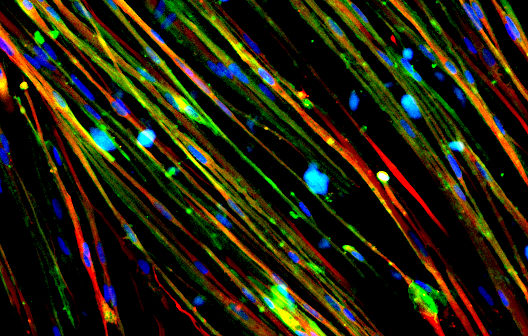
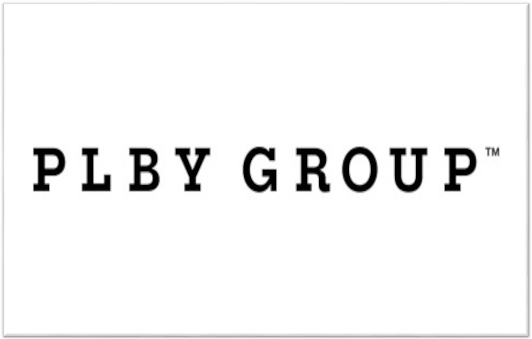Advancing Research into Alzheimer’s Disease with Stem Cells
Disease Burden of Alzheimer’s
Alzheimer’s disease (AD) is the most common cause of dementia, a progressively debilitating neurodegenerative disease that results in declining cognitive functions, inability to form new memories, behavioral disorders, and gradual loss of bodily functions. While older age does not cause AD, the risk of AD doubles about every five years beyond age 651. The World Health Organization estimates that about 50 million people living with dementia globally. In 2050, the number is expected to increase to 152 million2. In the U.S., a total of 122,019 recorded deaths were due to AD (2018), making it the 6th leading cause of death among adults in the country3. Hence, AD has a significant disease burden worldwide. Currently, the economic burden of AD is estimated to be $305 billion4. To date, there is no cure for dementia or AD.
Neuropathological Features and Causes of Alzheimer’s Disease
One of the key neuropathological features of AD is the presence of brain lesions consisting of amyloid plaques. It is caused by the pathological extracellular accumulation and deposition of amyloid-beta peptide (amyloid-beta)5. Although the exact function of amyloid-beta is largely unclear, there have been some speculations regarding its physiological roles in the body6. Regardless, targeting amyloid-beta production and accumulation remains to be an attractive therapeutic solution to treat AD7,8. Other hallmarks of AD include the presence of neurofibrillary tangles and hyperphosphorylated tau in brain tissues.
AD is a complex, multifactorial disease caused by a mix of genetic and environmental factors9. It can be broadly classified into two types – sporadic (SAD) and familial (FAD) AD. SAD and FAD are typically late-onset and early-onset respectively
10. FAD is rare and is caused by genetic mutations (such as in the Amyloid Precursor Protein [APP] gene); environmental factors play small roles in contributing to the disease. On the other hand, several genes are associated with SAD but environmental factors are also observed to contribute to the disease
9.
Lack of Suitable Platforms to Study AD
Over the past decade, researchers have adopted different systems to study AD. Post-mortem brain tissue samples from individuals with AD have been crucial in identifying and better understanding the physical and molecular changes of the brain caused by the disease. Cultured human and mouse cells have also been useful in understanding amyloid-beta accumulation and plaque formation but these cells are usually transformed to allow cells to remain proliferative; therefore they are not physiologically relevant and are unsuitable to study age-related aspects of AD. Also, it is difficult to grow and culture human neurons in the lab. Therefore, researchers looked to using transgenic mice to model AD. There have been some successes in generating FAD mice by overexpressing APP11. These mice were able to show age-related formation of amyloid-beta plaques, learning problems, and neuronal synapse loss. Today, there are more mice models that mimic various AD pathologies11. However, these mice are still unable to reflect the full complexity of the neurodegeneration process associated with AD12. The unfortunate reality is exemplified by the fact that clinical trials for AD drug development have a failure rate of 99.6%13.
Making Diseased Neurons in the Lab to Better Understand Disease Pathogenesis
The discovery of the Nobel Prize-winning iPSC technology
14,15 has advanced AD research by miles. There are many successes in using iPSCs to model and study both SAD and FAD in the lab16,17. Researchers have generated stem cell-derived neurons, microglia, astrocytes, and even 3D brain organoids that exhibited disease phenotypes. Because iPSCs are self-renewal and highly proliferative, we now have unlimited resources to study the disease pathogenesis of AD18.
Recently, a group of researchers from Harvard Medical School utilized the iPSC technology and reprogrammed skin cells from patients with SAD into iPSCs19. The patient-specific iPSCs were then differentiated into neural progenitor cells (NPCs) to obtain SAD NPCs. Using these diseased cells, they were able to uncover new information about the disease – diseased cells showed abnormal signs of accelerated maturation as compared to healthy cells. They were also discovered that the diseased cells have a loss of function of a protein called REST. By restoring levels of functional REST, they were able to prevent early maturation of SAD NPCs.
Another group of researchers from the Mayo Clinic differentiated AD iPSCs into cerebral organoids (‘mini-brains’), which are 3D self-organizing structures that recapitulate many features of the human brain. The diseased cerebral organoids showed high levels of amyloid-beta and phosphorylated tau, which are key features of AD. Using these diseased ‘mini-brains’, they were also able to uncover new knowledge about the disease which includes altered RNA metabolism and increased numbers of stress granules 20.
Stem-Cell Based Therapy for Alzheimer’s Disease
With the lack of suitable platforms to study AD as well as the gross limitations of current mice models of AD, researchers and the public alike have started to feel disheartened. However, in recent years, proof-of-concept studies of various types of stem cell-based therapies have shown great promise in treating AD.
Firstly, the use of Mesenchymal Stem Cells (MSCs) to treat AD is actively pursued right now. MSCs are stem cells that are found in umbilical cord blood, the Wharton jelly (a connective tissue found in the umbilical cord), bone marrow, and even fat tissues. In fact, a phase 1 clinical trial investigating the use of umbilical cord blood MSCs to treat AD patients was completed in 201521 and results showed that the treatment was both safe and feasible. MSCs can potentially treat AD via three ways – immune regulation, reducing numbers of amyloid-beta plaques, and promote neurotrophic regeneration and evidences suggest that the therapeutic potential of MSCs lies with the extracellular vesicles/ exosomes, produced by MSCs22. Exosomes contain nucleic acids, proteins, enzymes and even immune signals that are able to promote regeneration, regulation inflammation, and clear amyloid-beta plaques.
Next, the cellular transplantation of NPCs differentiated from embryonic stem cells (ESCs) was able to improve learning and memory functions in rat models of AD23. In addition, transplanting human induced-neural progenitor/ stem cells (iNPCs) into the hippocampus of AD mice restored cognitive deficits of diseased mice and significantly improved cognitive performance
24. Therefore, ESC-derived NPCs and iNPCs can potentially serve as therapeutic options to treat and improve cognitive functionalities of AD patients. Other than NPCs, stem cell-derived glial cells25 were also able to reduce amyloid-beta deposition and were effective in decreasing cognitive dysfunctions in AD mice.
The risk of graft rejection will be reduced if patient-specific iPSCs were used to generate cells for autologous transplantation. Genetic mutations found in the patients can also be edited via the CRISPR/Cas926 technology. This way, NPCs, neurons, or glial cells differentiated from patient-specific (edited) iPSCs will be healthy and will not be rejected by the patients’ immune system.
Concluding Comments
Over the past few years, research into AD has allowed us to better understand disease etiology and pathogenesis, but research efforts were hindered by the lack of suitable platforms to study the disease. Animal models of AD are still unable to fully reflect the complexities of the disease observed in humans. The advent of stem cell technologies has allowed us to model AD more accurately and sustainably. With that, stem cell-based therapies have also shown great successes in improving cognitive functions in animal models of AD. In the years to come, more studies would have to be done to determine the safety and efficacy of transplanting MSCs as well as ESC/iPSC-derived cells in treating AD patients.
About the Author: Nicole
Pek is a stem cell biologist and enthusiastic science communicator. She
has worked on using human pluripotent stem cells to study cellular development
in multiple organ systems, to model complex human diseases, and screen for
therapeutics that could treat the diseases. Outside of the lab, Nicole plays a
pro-active role in communicating to the public through her science blog ‘Two
Cells’ and her education podcast ‘The Diploid Duo’.
Suggested Reading:

|
|
The Anti-Aging and Rejuvenating Properties of Stem Cells
|
Therapeutic Discovery Advanced by Stem Cells
|
|
|
What Cells can be Made from Stem Cells
|
The Case for Investing in Regenerative Medicine
|
References
1. What Causes Alzheimer’s Disease? National Institute on Aging http://www.nia.nih.gov/health/what-causes-alzheimers-disease (2019).
2. Dementia. World Health Organization https://www.who.int/news-room/fact-sheets/detail/dementia (2020).
3. 2020 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia 16, 391–460 (2020).
4. Wong, W. Economic burden of Alzheimer disease and managed care considerations. Am J Manag Care 26, S177–S183 (2020).
5. Serrano-Pozo, A., Frosch, M. P., Masliah, E. & Hyman, B. T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb Perspect Med 1, (2011).
6. Brothers, H. M., Gosztyla, M. L. & Robinson, S. R. The Physiological Roles of Amyloid-beta Peptide Hint at New Ways to Treat Alzheimer’s Disease. Front Aging Neurosci 10, (2018).
7. Sevigny, J. et al. The antibody aducanumab reduces amyloid-beta plaques in Alzheimer’s disease. Nature 537, 50–56 (2016).
8. Panza, F., Lozupone, M., Logroscino, G. & Imbimbo, B. P. A critical appraisal of amyloid-beta-targeting therapies for Alzheimer disease. Nat Rev Neurol 15, 73–88 (2019).
9. Mayeux, R. & Stern, Y. Epidemiology of Alzheimer Disease. Cold Spring Harb Perspect Med 2, (2012).
10. Barber, R. C. The Genetics of Alzheimer’s Disease. Scientifica 2012, e246210 (2012).
11. Elder, G. A., Sosa, M. A. G. & Gasperi, R. D. Transgenic Mouse Models of Alzheimer’s Disease. Mount Sinai
Journal of Medicine: A Journal of Translational and Personalized Medicine 77, 69–81 (2010).
12. King, A. The search for better animal models of Alzheimer’s disease. Nature 559, S13–S15 (2018).
13. Cummings, J. Lessons Learned from Alzheimer Disease: Clinical Trials with Negative Outcomes. Clinical and
Translational Science 11, 147–152 (2018).
14. Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell
131, 861–872 (2007).
15. Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
16. Israel, M. A. et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells.
Nature 482, 216–220 (2012).
17. Ochalek, A. et al. Neurons derived from sporadic Alzheimer’s disease iPSCs reveal elevated TAU hyperphosphorylation, increased amyloid levels, and GSK3B activation. Alzheimers
Res Ther 9, 90 (2017).
18. de Leeuw, S. & Tackenberg, C. Alzheimer’s in a dish – induced pluripotent stem cell-based disease modeling. Translational
Neurodegeneration 8, 21 (2019).
19. Meyer, K. et al. REST and Neural Gene Network Dysregulation in iPSC Models of Alzheimer’s Disease. Cell
Reports 26, 1112-1127.e9 (2019).
20. Zhao, J. et al. APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. Nat Commun 11, 5540 (2020).
21. Kim, H. J. et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: A phase 1 clinical trial. Alzheimers
Dement (N Y) 1, 95–102 (2015).
22. Elia, C. A., Losurdo, M., Malosio, M. L. & Coco, S. Extracellular Vesicles from Mesenchymal Stem Cells Exert Pleiotropic Effects on Amyloid-beta, Inflammation, and Regeneration: A Spark of Hope for Alzheimer’s Disease from Tiny Structures? BioEssays 41, 1800199 (2019).
23. Tang, J. et al. Embryonic stem cell-derived neural precursor cells improve memory dysfunction in amyloid-beta(1-40) injured rats. Neurosci Res 62, 86–96 (2008).
24. Zhang, T. et al. Human Neural Stem Cells Reinforce Hippocampal Synaptic Network and Rescue Cognitive Deficits in a Mouse Model of Alzheimer’s Disease. Stem Cell Reports 13, 1022–1037 (2019).
25. Cha, M.-Y. et al. Protein-Induced Pluripotent Stem Cells Ameliorate Cognitive Dysfunction and Reduce amyloid-beta Deposition in a Mouse Model of Alzheimer’s Disease. Stem Cells Transl Med
6, 293–305 (2017).
26. Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. Nature Protocols 8, 2281–2308 (2013).
Stay up to date. Follow us:
 |
 |
 |
 |
 |
 |
Stay up to date. Follow us:
 |
 |
 |
 |
 |
 |