image credit: U.S. Federal Reserve
The Federal Reserve’s Discussion Paper on Digital Currency to be Released this Summer
Last week, Fed Chair Jerome Powell spoke in a video address about CBDCs (Central Bank Digital Currencies) and other cryptocurrencies. He announced the Federal Reserve plans to publish a discussion paper on the potential benefits and risks of issuing a U.S. digital currency during this summer. He also shared more insight on current thinking.
“The paper represents the beginning of what will be a thoughtful and deliberative process,” said Chairman Powell in his address. He also spoke of regulation making it clear there were no decisions made to date when expressing the eye in which the Fed will look at the matter, “ the Federal Reserve is charged with promoting monetary and financial stability and the safety and efficiency of the payment system. In pursuit of these core functions, we have been carefully monitoring and adapting to the technological innovations now transforming the world of payments, finance, and banking.” Powell also made clear that one decision that has been made is that cash currency is not going away, “We think it is important that any potential CBDC could serve as a complement to, and not a replacement of, cash and current private-sector digital forms of the dollar, such as deposits at commercial banks,” said Powell.
A link for the video is provided below as a “Source,” but, as with most Fed releases, every word is deliberate and therefore worth reading. Here is the complete transcript.
Transcript of Chair Powell’s Message on Developments in the U.S. Payments System
May 20, 2021
Today we are in the midst of a technological revolution that is fundamentally changing our world: reshaping how we communicate, access information, and purchase goods and services. As the central bank of the United States, the Federal Reserve is charged with promoting monetary and financial stability and the safety and efficiency of the payment system. In pursuit of these core functions, we have been carefully monitoring and adapting to the technological innovations now transforming the world of payments, finance, and banking.
The effective functioning of our economy requires that people have faith and confidence not only in the dollar, but also in the payment networks, banks, and other payment service providers that allow money to flow on a daily basis. Our focus is on ensuring a safe and efficient payment system that provides broad benefits to American households and businesses while also embracing innovation. In the Fed’s early days, the development of dedicated telegraph wires facilitated the transfer of funds between banks. In the 1980s, automated clearinghouse services made it possible for electronic bill payments to be an alternative to paper checks. In 2019, the Federal Reserve committed to building the FedNow Service, which will enable banks to provide real-time, or instant, payments to their customers around the clock, 365 days a year, benefiting communities across America.
Recently, the rise of distributed ledger technology, which offers a new approach to recording ownership of assets, has allowed for the creation of a range of new financial products and services—including cryptocurrencies. To date, cryptocurrencies have not served as a convenient way to make payments, given, among other factors, their swings in value. Nonetheless, coins tied to the value of the dollar or another currency—known as “stablecoins”— have emerged as a new way to make payments. These stablecoins aim to use new technologies in a way that has the potential to enhance payments efficiency, speed up settlement flows, and reduce end-user costs—but they may also carry potential risks to those users and to the broader financial system. For example, although the value of a stablecoin may be tied to the value of a dollar, these coins may not come with the same protections as traditional means of payment, such as physical currency or the deposits in your bank account. Therefore, as stablecoins’ use increases, so must our attention to the appropriate regulatory and oversight framework. This includes paying attention to private-sector payments innovators who are currently not within the traditional regulatory arrangements applied to banks, investment firms, and other financial intermediaries.
Technological advances also offer new possibilities to central banks—including the Fed. In particular, technology now enables the development and issuance of central bank digital currencies, or CBDCs. A CBDC is a new type of central bank liability issued in digital form. While various structures and technologies might be used, a CBDC could be designed for use by the general public.
For the past several years, the Federal Reserve has been exploring the potential benefits and risks of CBDCs from a variety of angles, including through technological research and experimentation. Our key focus is on whether and how a CBDC could improve on an already safe, effective, dynamic, and efficient U.S. domestic payments system. We think it is important that any potential CBDC could serve as a complement to, and not a replacement of, cash and current private-sector digital forms of the dollar, such as deposits at commercial banks. The design of a CBDC would raise important monetary policy, financial stability, consumer protection, legal, and privacy considerations and will require careful thought and analysis— including input from the public and elected officials.
To help stimulate broad conversation, the Federal Reserve Board will issue a discussion paper this summer outlining our current thinking on digital payments, with a particular focus on the benefits and risks associated with CBDC in the U.S. context. As part of this process, we will ask for public comment on issues related to payments, financial inclusion, data privacy, and information security.
We are committed at the Federal Reserve to hearing a wide range of voices on this important issue before making any decision on whether and how to move forward with a U.S. CBDC, taking account of the broader risks and opportunities it could offer. The paper represents the beginning of what will be a thoughtful and deliberative process. Irrespective of the conclusion we ultimately reach, we expect to play a leading role in developing international standards for CBDCs, engaging actively with central banks in other jurisdictions as well as regulators and supervisors here in the United States throughout that process.
The Federal Reserve remains committed to ensuring that the public has access to a safe, reliable, and secure payments system. Our forthcoming paper on the evolution of digital payments is intended—along with our other work as a supervisor, regulator, and payment system operator—to advance the objective of ensuring that the payments system and the economy work for all Americans. We look forward to hearing your thoughts on this important topic.
Take-Away
In his announcement, Chairman Powell showed he understood how technology in the past has led to better communication, faster payment systems, and more efficient bank clearing operations. He also was clear that, to date, the experience with cryptocurrencies is that they do not serve as a convenient way to make payments, “given, among other factors, their swings in value.” Adopting new technologies such as distributed ledger systems and a stable payment system is the balance the Fed will likely try to create going forward.
We will know more when the discussion paper is released later this year; in the interim, cryptocurrency exchange rates are likely to move up and down on, among other things, speculation of the tone of the upcoming paper.
Suggested Reading:

|

|
Small-Cap Names in a Big Crypto Market
|
Backed by the Full Faith and Credit of Blockchain
|

|

|
Cryptocurrency and the Howey Test
|
NFTs are Becoming More Popular with Sports Fans
|
Sources:
Chairman Powell’s Video Message May 20, 2020
Stay up to date. Follow us:
 |
 |
 |
 |
 |
 |
Stay up to date. Follow us:
 |
 |
 |
 |
 |
 |



























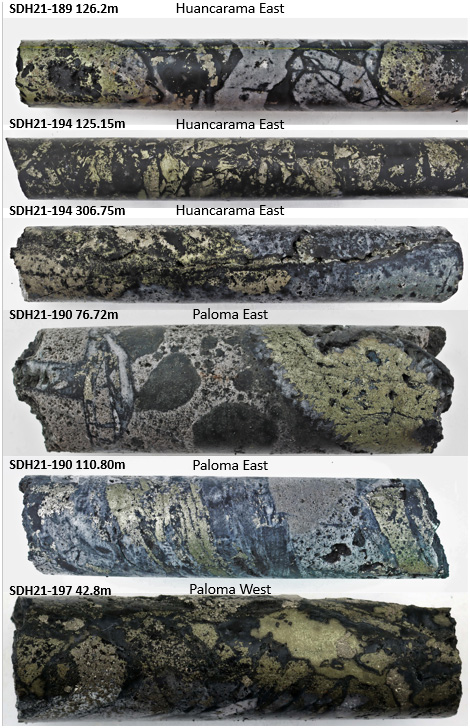 Figure 4 – Select core photos from Huancarama, Paloma East, and Paloma West reported in this release: SDH21-189 (126.2m) mosaic and jigsaw tourmaline breccia with sulfide cement; SDH21-194 (125.15m) mosaic breccia with selective clast replacement by chalcopyrite and pyrite; SDH21-194 306.75m tourmaline breccia with late chalcopyrite replacement along vein; SDH21-190 (76.72m) tourmaline breccia with chalcopyrite filling open space; SDG21-190 (110.80) shingle breccia with selective chalcopyrite replacement of shingle clasts; SDH21-197 (42.8m) mosaic tourmaline breccia with selective clast replacement and open space filling by chalcopyrite and pyrite. Core diameter is 6.35cm (HQ) in all instances.
Figure 4 – Select core photos from Huancarama, Paloma East, and Paloma West reported in this release: SDH21-189 (126.2m) mosaic and jigsaw tourmaline breccia with sulfide cement; SDH21-194 (125.15m) mosaic breccia with selective clast replacement by chalcopyrite and pyrite; SDH21-194 306.75m tourmaline breccia with late chalcopyrite replacement along vein; SDH21-190 (76.72m) tourmaline breccia with chalcopyrite filling open space; SDG21-190 (110.80) shingle breccia with selective chalcopyrite replacement of shingle clasts; SDH21-197 (42.8m) mosaic tourmaline breccia with selective clast replacement and open space filling by chalcopyrite and pyrite. Core diameter is 6.35cm (HQ) in all instances.