Palladium One Drills Haukiaho Trend, Intercepts 72 Meters @ 1.8 g/t Palladium-Equivalent, Finland
May 26, 2021 – Toronto, Ontario – First drill results from the Company’s 2,000 meter drill program at Haukiaho, a zone approximately 20 kilometers south of the Company’s primary target area Kaukua South, have returned significant widths and grades, including 72 meters at 1.8 g/t Palladium-equivalent “Pd_Eq”) (Hole LK21-071), on the Läntinen Koillismaa “LK”) PGE-Ni-Cu project in Finland, said Palladium One Mining Inc. “Palladium One” or the “Company”) (TSXV: PDM, FRA: 7N11, OTC: NKORF) today.
A total of 12 holes (1,943 metres) were drilled on the Haukiaho trend. This release contains the results from the first 7 holes of this program. The drilling was designed establish sufficient drill density to prepare a National Instrument 43-101 resource estimate at Haukiaho, which the Company anticipates completing in Q3 2021.
“Establishing a NI43-101 resource estimate at Haukiaho has been a priority for the Company given the robust historical data set available to the Company. Haukiaho was the original focus of the Phase I drill program in 2020, however, the discovery of Kaukua South led us towards first defining the potential of this new discovery and exploring the greater Kaukua area. Despite this, Haukiaho was not forgotten and has remained an important part of our strategy to grow a multi-million ounce resource base. At 17 kilometers in length, the Haukiaho trend, currently represents the largest continuous patch of blue-sky potential on the LK property.” said Derrick Weyrauch, President and CEO.
Highlights
- Drilling has returned significant widths and grades
- Expanded higher grade areas within the known historic Haukiaho resource area
- Expanded mineralization 100m to east of the historic resource
- 72.2 meters grading 1.79 g/t Pd_Eq in hole LK21-071 starting 56m down hole, including 17.0 meters grading 2.23 g/t Pd_Eq
- 15.7 meters grading 2.26 g/t Pd_Eq in hole LK21-069 including 3.6 meters grading 3.11 g/t Pd_Eq
- 51.2 meters grading 1.07 g/t Pd_Eq in hole LK21-073 including 9.1 meters grading 2.10 g/t Pd_Eq
- The historic Haukiaho resource is shallowly drilled, mostly above 200m in depth and therefore amendable to open pit mining
Haukiaho Historic Resource Estimate
In the 1980’s, Outokumpu (a Finnish State-run mining company) prepared a resource estimate using very widely spaced holes along a portion of the Haukiaho trend, which estimated 7 million tonnes grading 0.38% Cu and 0.24% Ni, however importantly, no PGE assays were undertaken. The cut-off grade used was a 0.7% Copper_equivalent (defined as Cu% + 2 x Ni%).
Subsequently in 2013, Finore Mining Inc. completed a non-pit constrained NI43-101 historic resource estimate, over a much smaller strike length. Using a 0.1 g/t Pd cut-off, they estimated a resource of 1.13 million Pd_Eq ounces within 23.2 million tonnes grading 1.51 g/t Pd_Eq (0.31 g/t Pd, 0.12g/t Pt, 0.10 g/t Au, 0.21% Cu, and 0.14% Ni) (See news release August 12, 2019 and May 7, 2020). This resource estimate encompassed widely spaced drilling with a focus on maximizing tonnage, not grade.
The Haukiaho trend is 17 kilometers long and the historic 2013 Haukiaho resource prepared by Finore covers less than 2 kilometers of this trend. This knowledge coupled with the historic work by Outokumpu point to the enormous potential to significantly add resources at Haukiaho with disciplined execution of exploration activities.
While similar to the Kaukua deposit (See new release, September 30, 2019), the Haukiahio trend is more copper-nickel rich. At Kaukua, approximately ~1/3 of the in-situ value per tonne is copper-nickel, while at Haukiaho copper-nickel represent approximately ~2/3s of the in-situ value.
Figure 1. LK Project location map showing 43-101 compliant Kaukua deposit and historic Haukiaho resource along with 2020 IP grids (blue lines) and current 2021 IP grid areas (black boxes). Yellow lines represent Exploration Permits, red lines represent Exploration Reservations held by the Company.
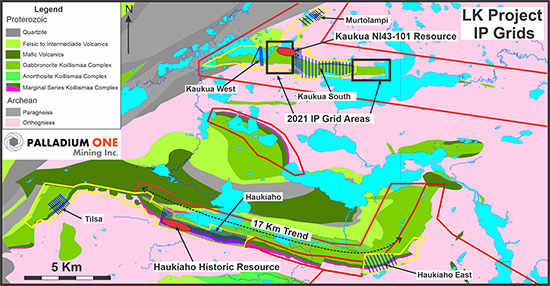
Figure 2. Plan map of Haukiaho 2020 IP chargeability anomalies and Palladium One drill holes locations.

Figure 3. Plan map of the Haukiaho historic 2013 Finore resource estimate represented by the > 0.5g/t Pd_Eq resource shape with Palladium One drill holes.
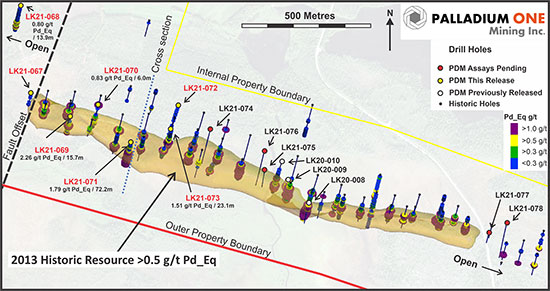
Figure 4. Haukiaho Cross section showing holes LK21-071, Finore hole HAU11-005, and geological survey of Finland (GTK) holes R-390 and R-389.
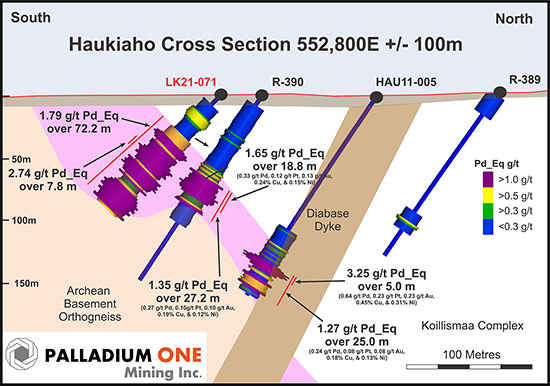
Table 1: Palladium One Haukiaho drill results
|
Hole
|
From
(m)
|
To
(m)
|
Width
(m)
|
Pd_Eq
g/t*
|
Pd
g/t
|
Pt
g/t
|
Au
g/t
|
Cu
%
|
Ni
%
|
|
LK20-008
|
17.3
|
33.5
|
16.2
|
1.99
|
0.38
|
0.15
|
0.14
|
0.26
|
0.20
|
|
Inc.
|
20.3
|
23.3
|
3.0
|
2.55
|
0.48
|
0.19
|
0.22
|
0.33
|
0.25
|
|
LK20-009
|
161.5
|
168.1
|
6.6
|
2.34
|
0.47
|
0.20
|
0.13
|
0.26
|
0.25
|
|
LK20-010
|
118.7
|
202.0
|
83.3
|
1.27
|
0.24
|
0.09
|
0.05
|
0.12
|
0.16
|
|
Inc.
|
166.8
|
201.0
|
34.3
|
2.09
|
0.47
|
0.20
|
0.10
|
0.20
|
0.22
|
|
Inc.
|
167.8
|
173.0
|
5.3
|
3.08
|
0.66
|
0.25
|
0.20
|
0.38
|
0.30
|
|
Inc.
|
20.3
|
23.3
|
3.0
|
2.55
|
0.48
|
0.19
|
0.22
|
0.33
|
0.25
|
|
LK21-067
|
No Significant values (collared in footwall rocks)
|
|
LK21-068
|
81.5
|
122.0
|
40.6
|
0.65
|
0.05
|
0.02
|
0.02
|
0.04
|
0.11
|
|
Inc.
|
106.2
|
120.0
|
13.9
|
0.80
|
0.12
|
0.05
|
0.02
|
0.06
|
0.12
|
|
Inc.
|
106.2
|
112.7
|
6.6
|
0.91
|
0.13
|
0.05
|
0.03
|
0.09
|
0.13
|
|
LK21-069
|
48.5
|
64.1
|
15.7
|
2.26
|
0.47
|
0.18
|
0.18
|
0.27
|
0.22
|
|
Inc.
|
48.5
|
60.0
|
11.6
|
2.53
|
0.52
|
0.19
|
0.18
|
0.30
|
0.25
|
|
Inc.
|
48.5
|
52.0
|
3.6
|
3.11
|
0.60
|
0.22
|
0.23
|
0.37
|
0.32
|
|
LK21-070
|
103.5
|
131.0
|
27.5
|
0.65
|
0.01
|
0.00
|
0.02
|
0.04
|
0.12
|
|
Inc.
|
113.0
|
119.0
|
6.0
|
0.83
|
0.02
|
0.00
|
0.04
|
0.09
|
0.14
|
|
LK21-071
|
55.8
|
128.0
|
72.2
|
1.79
|
0.37
|
0.15
|
0.14
|
0.22
|
0.17
|
|
Inc.
|
72.0
|
77.0
|
5.0
|
2.33
|
0.53
|
0.21
|
0.18
|
0.27
|
0.22
|
|
Inc.
|
74.0
|
75.5
|
1.5
|
3.20
|
0.75
|
0.29
|
0.23
|
0.39
|
0.29
|
|
And
|
86.2
|
94.0
|
7.8
|
2.74
|
0.53
|
0.21
|
0.23
|
0.32
|
0.27
|
|
And
|
111.0
|
128.0
|
17.0
|
2.23
|
0.53
|
0.21
|
0.18
|
0.28
|
0.18
|
|
Inc.
|
112.5
|
118.0
|
5.5
|
2.84
|
0.64
|
0.25
|
0.23
|
0.36
|
0.25
|
|
LK21-072
|
No Significant values (ended before zone)
|
|
LK21-073
|
75.8
|
127.0
|
51.2
|
1.07
|
0.15
|
0.06
|
0.06
|
0.13
|
0.13
|
|
Inc.
|
90.0
|
113.1
|
23.1
|
1.51
|
0.25
|
0.10
|
0.10
|
0.21
|
0.16
|
|
Inc.
|
90.8
|
99.9
|
9.1
|
2.10
|
0.37
|
0.15
|
0.16
|
0.27
|
0.21
|
|
Inc.
|
98.0
|
99.0
|
1.0
|
3.21
|
0.61
|
0.27
|
0.29
|
0.47
|
0.28
|
|
LK21-074
|
Pending
|
|
LK21-075
|
Pending
|
|
LK21-076
|
Pending
|
|
LK21-077
|
Pending
|
|
LK21-078
|
Pending
|
* Reported widths are “drilled widths” not true widths.
**Italicised orange highlighted results are previously released results see news release September 15, 2020
*Palladium Equivalent
Palladium equivalent is calculated using US$1,100 per ounce for palladium, US$950 per ounce for platinum, US$1,300 per ounce for gold, US$6,614 per tonne for copper, and US$15,4332 per tonne for nickel. This calculation is consistent with the calculation in the Company’s September 2019 NI 43-101 Kaukua resource estimate. Additionally, US$1,100 per ounce for palladium is consistent with the UBS January 2021 long-term consensus price forecast even though the current price of palladium is approximately US$2,800 per ounce.
QA/QC
The Phase I drilling program was carried out under the supervision of Neil Pettigrew, M.Sc., P. Geo., Vice President of Exploration and a director of the Company.
Drill core samples were split using a rock saw by Company staff, with half retained in the core box and stored indoors in a secure facility, in Taivalkoski, Finland. The drill core samples were transported by courier from the Company’s core handling facility in Taivalkoski, Finland, to ALS Global “ALS”) laboratory in Outokumpu, Finland. ALS, is an accredited lab and are ISO compliant (ISO 9001:2008, ISO/IEC 17025:2005). PGE analysis was performed using a 30 grams fire assay with an ICP-MS or ICP-AES finish. Multi-element analyses, including copper and nickel were analysed by four acid digestion using 0.25 grams with an ICP-AES finish.
Certified standards, blanks and crushed duplicates are placed in the sample stream at a rate of one QA/QC sample per 10 core samples. Results are analyzed for acceptance at the time of import. All standards associated with the results in this press release were determined to be acceptable within the defined limits of the standard used.
Qualified Person
The technical information in this release has been reviewed and verified by Neil Pettigrew, M.Sc., P. Geo., Vice President of Exploration and a director of the Company and the Qualified Person as defined by National Instrument 43-101.
About Palladium One
Palladium One Mining Inc. is an exploration company targeting district scale, platinum-group-element (PGE)-copper nickel deposits in Finland and Canada. Its flagship project is the Läntinen Koillismaa or LK Project, a palladium dominant platinum group element-copper-nickel project in north-central Finland, ranked by the Fraser Institute as one of the world’s top countries for mineral exploration and development. Exploration at LK is focused on targeting disseminated sulfides along 38 kilometers of favorable basal contact and building on an established NI 43-101 open pit resource.
ON BEHALF OF THE BOARD
“Derrick Weyrauch”
President & CEO, Director
For further information contact: Derrick Weyrauch, President & CEO
Email: info@palladiumoneinc.com
Neither the TSX Venture Exchange nor its Market Regulator (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
This press release includes “forward-looking information” that is subject to a few assumptions, risks and uncertainties, many of which are beyond the control of the Company. Statements regarding listing of the Company’s common shares on the TSXV are subject to all of the risks and uncertainties normally incident to such events. Investors are cautioned that any such statements are not guarantees of future events and that actual events or developments may differ materially from those projected in the forward-looking statements. Such forward-looking statements represent management’s best judgment based on information currently available. Factors that could cause the actual results to differ materially from those in forward-looking statements include regulatory actions and general business conditions. Such forward-looking information reflects the Company’s views with respect to future events and is subject to risks, uncertainties and assumptions, including those set out in the Company’s annual information form dated April 29, 2020 and filed under the Company’s profile on SEDAR at www.sedar.com. The Company does not undertake to update forward?looking statements or forward?looking information, except as required by law. Investors are cautioned that any such statements are not guarantees of future performance and actual results or developments may differ materially from those projected in the forward-looking statements.