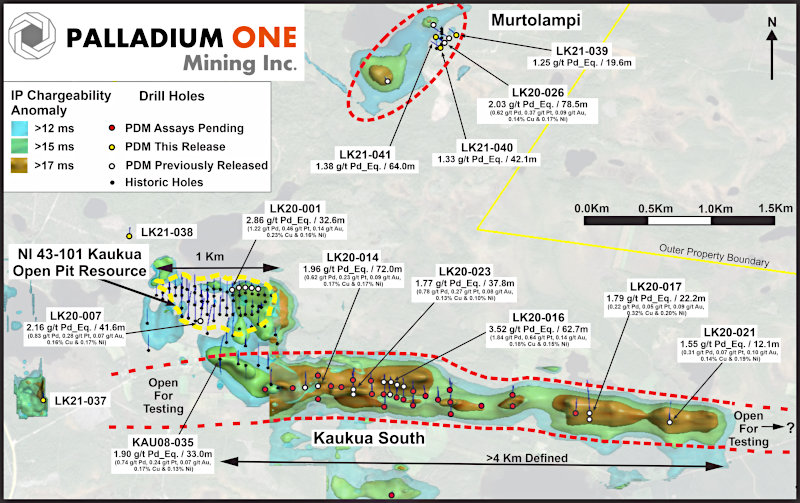
Chakana Copper Extends Huancarama Mineralization 290m Below Surface – Soledad Project, Peru
Vancouver, B.C., March 3, 2021 – Chakana Copper Corp. (TSX-V: PERU; OTCQB: CHKKF; FRA: 1ZX) (the “Company” or “Chakana”), is pleased to release results for two additional drill holes from the Huancarama Breccia Complex, within the Soledad Project in Ancash, Peru (Fig. 1). One hole (SDH20-165) was designed to test beneath the exposed H4 breccia, and a second hole (SDH20-166) was designed to explore a deeper extent of the Huancarama East breccia discovery (see figure 2, and news releases dated January 12, 25, and February 9, 2021). Since initiating drilling at Huancarama in late October 2020, thirty HQ diamond core holes have been completed.
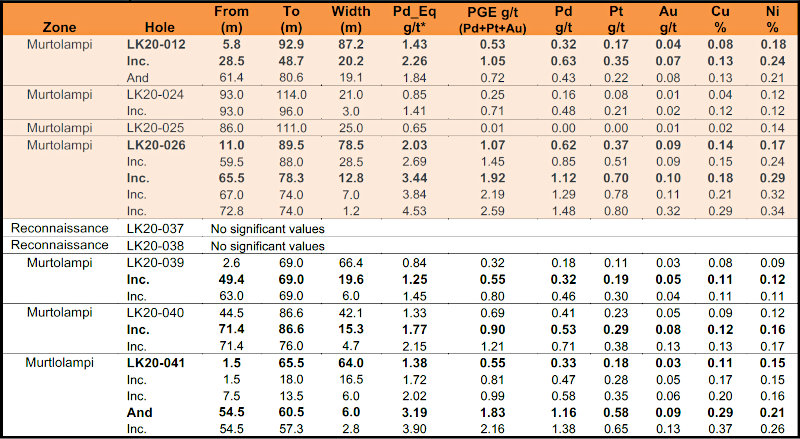
Mineralized intervals from two additional holes at Huancarama include:
* Cu_eq and Au_eq values were calculated using copper, gold, and silver. Metal prices utilized for the calculations are Cu – US$2.90/lb, Au – US$1,300/oz, and Ag – US$17/oz. No adjustments were made for recovery as the project is an early-stage exploration project and metallurgical data to allow for estimation of recoveries are not yet available. The formulas utilized to calculate equivalent values are Cu-eq (%) = Cu% + (Au g/t * 0.6556) + (Ag g/t * 0.00857) and Au-eq (g/t) = Au g/t + (Cu% * 1.5296) + (Ag g/t * 0.01307).
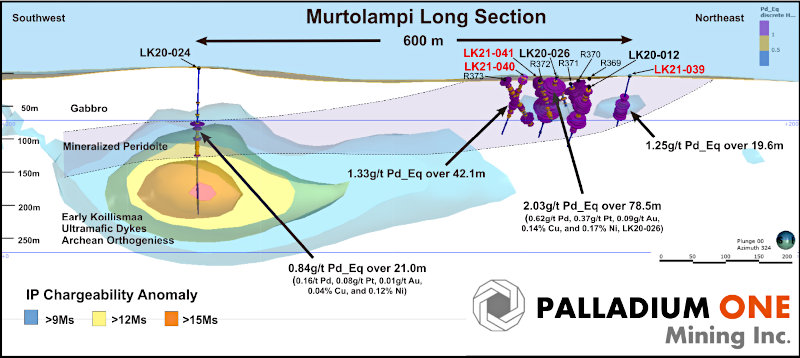
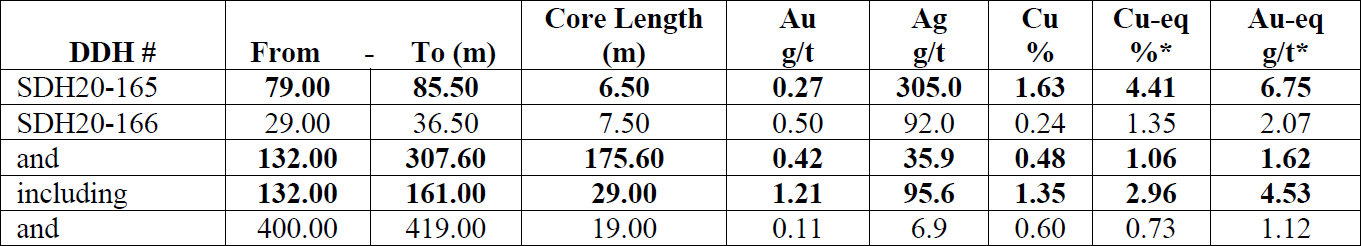
Both drill holes intercepted high-grade intervals (Figs. 2 and 3). Hole SDH20-165 was set up southeast of the H4 surface breccia and drilled to the northwest encountering a high-grade breccia interval of 0.27 g/t Au, 1.63% Cu, and 305.0 g/t Ag (4.41% Cu-eq) over 6.5m starting at 79m depth. This intercept is believed to be related to the H4 breccia exposed at surface and is interpreted to be a breccia dike, related to a larger, adjacent breccia body. Breccia dikes adjacent to larger breccia pipes are a common feature of the breccia pipes at Soledad.
Hole SDH20-166 was set up on the east side of the main Huancarama East breccia pipe and drilled steeply across the pipe in a west-northwest direction. This hole entered the main breccia body at 107.2m down hole (102m below surface) and exited the breccia pipe on the northwest side at 308.2m depth (290m below surface). A continuous mineralized interval of 175.6m with 0.42 g/t Au, 0.48% Cu, and 35.9 g/t Ag (1.06% Cu-eq) was encountered starting at 132.0m. Within this interval, a high-grade zone of 1.21 g/t Au, 1.35% Cu, and 95.6 g/t Ag (2.96% Cu-eq) over 29.0m starting at 132.0m depth was also encountered. Two additional breccia intercepts, also interpreted as breccia dikes related to the Huancarama East breccia pipe, were encountered at 402.7m and 483.4m depth, with the breccia pipe open at depth. Examples of mineralized drill core from these holes are shown in Figure 4.
David Kelley, President and CEO commented, “Hole SDH20-166 was successful in increasing the Huancarama East
breccia pipe to a depth of 290m below surface with mineralization open at depth. We had previously established
mineralization starting at surface and continuing to a depth of approximately 225m depth. As we have seen in the
other breccia pipe discoveries, these pipes are vertically extensive, and all are still open at depth. The high-grade
intercept of 29.0m with 1.21 g/t Au, 1.35% Cu, and 95.6 g/t Ag is particularly noteworthy as it provides additional
confirmation of the high-grade potential of this new discovery and is consistent with intercepts recently reported. It is
also encouraging to encounter high-grade mineralization in the first hole drilled around H4 on the western side of the complex. As we have seen in drilling other breccia pipes at Soledad, these high-grade intercepts are normally related
to adjacent larger mineralized breccia bodies. More drilling is warranted on the western side of Huancarama. and
drilling is ongoing at both Huancarama and Paloma with two drill rigs; we look forward to reporting additional results
soon.”
Huancarama Target Area and the Current Drill Program
The Huancarama Breccia Complex is located 300m south of and 400m above the deepest breccia intercept at Paloma. Within the complex there are five principal breccia bodies exposed at surface over approximately 200m horizontally (Fig. 5). There is a distinctive feature believed to be a collapse zone with dimensions of 50m by 30m. Unverified reports suggest that this may be due to small-scale mining. Two historic adits are in the complex, one trending north-northeast for 170m along the western side of H1 (Fig. 2), and a second shorter adit of 21m at H2. Surface sampling from the breccia bodies and channel sampling of the adits yielded strongly anomalous gold results (see news release dated November 19, 2019). In addition to several targets within the complex, numerous additional targets exist in the Huancarama and Paloma area.
Results reported here are part of the recently expanded 2021 drill program of 26,000m that is fully funded from the Company’s current treasury of $9.3M. Combined with the drilling in 2020 that started last August, a total of approximately 32,000m is anticipated through 2021. Of this, 6,634m have been reported in 34 drill holes. For the 26,000m of drilling planned in 2021, the Company will complete 16,000m of resource definition drilling, and 10,000m of exploration drilling testing new targets. This drill program and additional targets will be integral to the publication of a maiden resource in 2021.
Corporate Update
The Company announces that its second tranche of financing announced February 10, 2021 has been extended in order to facilitate the receipt of funds already committed over the next few days.
About Chakana Copper
Chakana Copper Corp is a Canadian-based minerals exploration company that is currently advancing the high-grade gold-copper-silver Soledad Project located in the Ancash region of Peru, a highly favorable mining jurisdiction with supportive communities. The Soledad Project consists of high-grade gold-copper-silver mineralization hosted in tourmaline breccia pipes. A total of 33,353 metres of drilling has been completed to-date, testing nine (9) of twenty-three (23) confirmed breccia pipes with more than 92 total targets. Chakana’s investors are uniquely positioned as the Soledad Project provides exposure to several metals including copper, gold, and silver. For more information on the Soledad project, please visit the website a www.chakanacopper.com.
Sampling and Analytical Procedures
Chakana follows rigorous sampling and analytical protocols that meet or exceed industry standards. Core samples are stored in a secured area until transport in batches to the ALS facility in Callao, Lima, Peru. Sample batches include certified reference materials, blank, and duplicate samples that are then processed under the control of ALS. All samples are analyzed using the ME-MS41 (ICP technique that provides a comprehensive multi-element overview of the rock geochemistry), while gold is analyzed by AA24 and GRA22 when values exceed 10 g/t by AA24. Over limit silver, copper, lead and zinc are analyzed using the OG-46 procedure. Soil samples are analyzed by 4-acid (ME-MS61) and for gold by Fire Assay on a 30g sample (Au-ICP21).
Results of previous drilling and additional information concerning the Project, including a technical report prepared in accordance with National Instrument 43-101, are made available on Chakana’s SEDAR profile at www.sedar.com.
Qualified Person
David Kelley, an officer and a director of Chakana, and a Qualified Person as defined by NI 43-101, reviewed and approved the technical information in this news release.
ON BEHALF OF THE BOARD
(signed) “David Kelley”
David Kelley
President and CEO
For further information contact:
Joanne Jobin, Investor Relations Officer
Phone: 647 964 0292
Email: jjobin@chakanacopper.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward-looking Statement Advisory: This release may contain forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties, and other factors which may cause the actual results, performance, or achievements of Chakana to be materially different from any future results, performance, or achievements expressed or implied by the forward looking statements. Forward looking statements or information relates to, among other things, the interpretation of the nature of the mineralization at the Soledad copper-gold-silver project (the “Project”), the potential to expand the mineralization, and to develop and grow a resource within the Project, the planning for further exploration work, the ability to de-risk the potential exploration targets, and our belief in the potential for mineralization within unexplored parts of the Project. These forward-looking statements are based on management’s current expectations and beliefs but given the uncertainties, assumptions and risks, readers are cautioned not to place undue reliance on such forward- looking statements or information. The Company disclaims any obligation to update, or to publicly announce, any such statements, events or developments except as required by law.
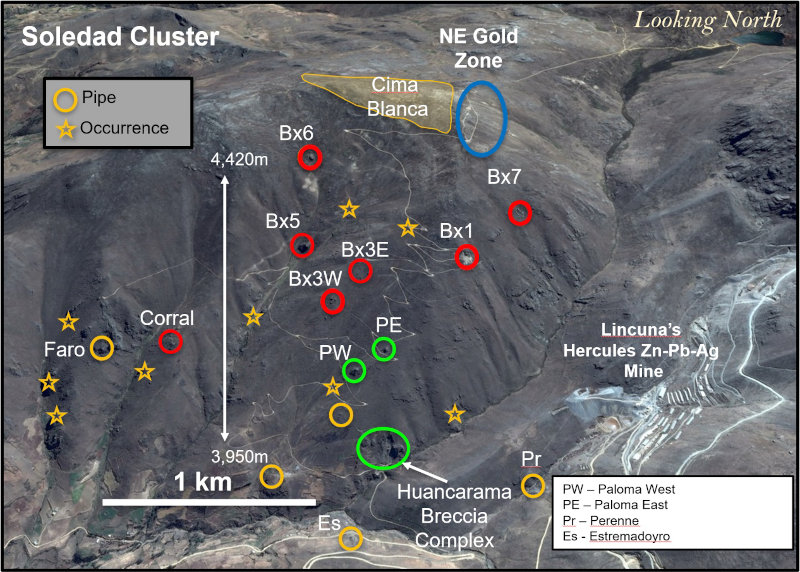
Figure 1 – View looking north showing breccia pipes and occurrences within the northern Soledad cluster. Pipes that have been drilled in previous campaigns are shown in red. Outcropping breccia pipes shown in green are the focus of the current drill campaign. Other pipes and occurrences remain to be tested by drilling. Additional breccia pipes occur on the south half of the property and are not shown here.
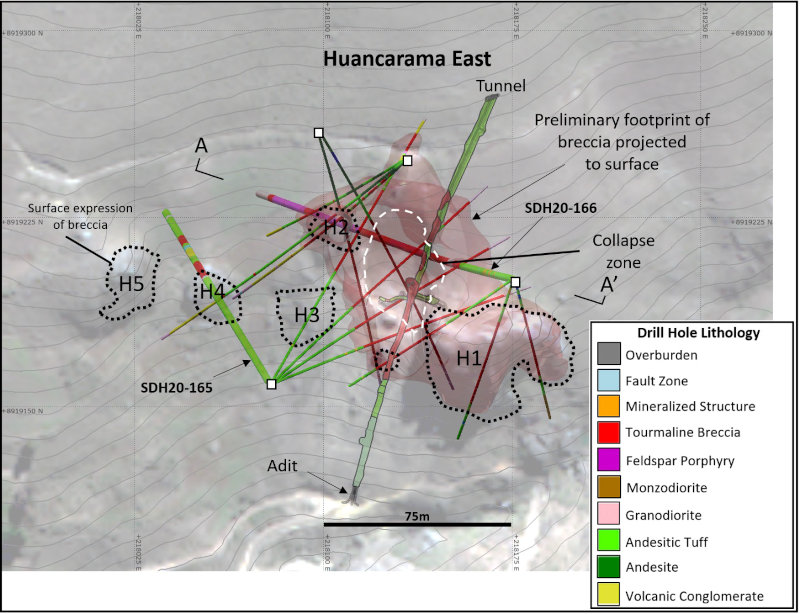
Figure 2 – Map of the Huancarama Breccia Complex and drill hole lithology in holes completed to date. Red shape projected to surface represents tourmaline breccia pipe based on all holes drilled to date and lithology mapped in the underground tunnel. Black dotted outlines show surface expression of mapped breccias (H1-H5); white dashed line shows collapse zone. Location of section line for Figure 3 indicated.
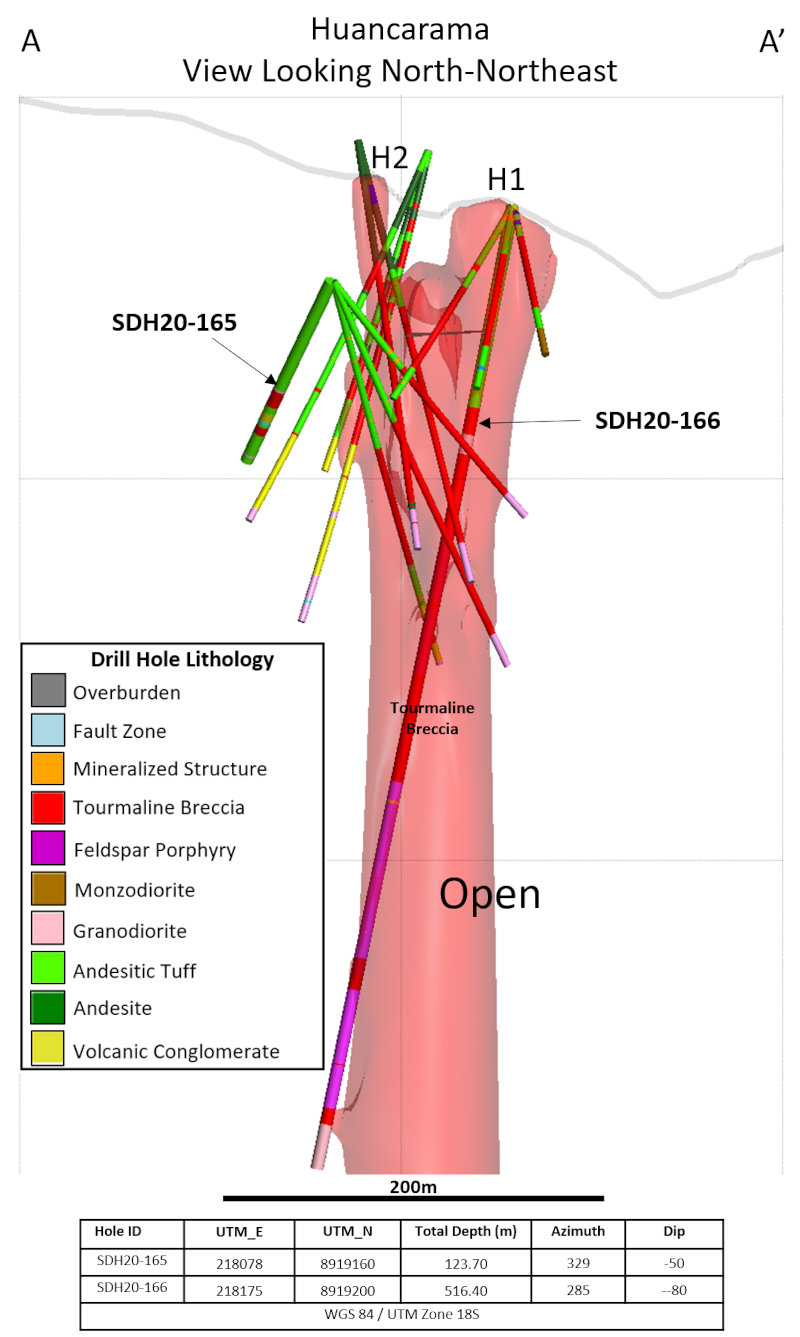
Figure 3 – Section looking north-northeast highlighting the drill holes at Huancarama reported in this release. Light red 3D shape shows preliminary shape of breccia based on all drill holes to date and lithology mapped in the underground tunnel.

Figure 4 – Core photos from Huancarama: SDH20-165 (83.0m) tourmaline breccia with semi-massive chalcopyrite replacement; SDH20-166 (128.43m to 136.25m) contact between feldspar porphyry (earlier) and tourmaline breccia with sections of massive sulfide replacement by chalcopyrite and pyrite. Core diameter is 6.35cm (HQ) in all instances.
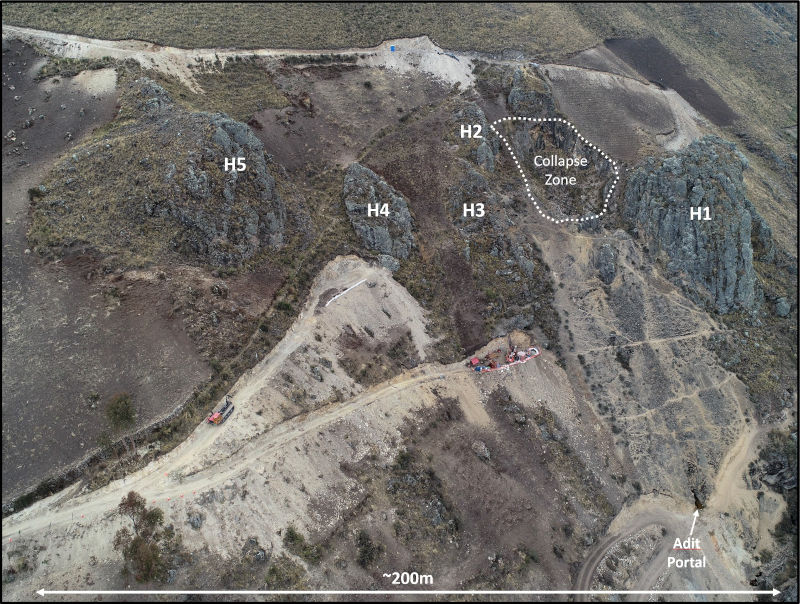
Figure 5 – Drone image looking northeast at the Huancarama Breccia Complex showing the five principal tourmaline breccia bodies exposed at surface (H1-H5), historic adit portal, and drill platforms. Note drill rig in center of image.
SOURCE: Chakana Copper