|
|
|
Kiromic Biopharma (KRPB) CEO Maurizio Chiriva-Internati at NobleCon17 – Noble Capital Markets 17th Annual Small & Microcap Investor Conference – January 2021. Following the formal presentation, Noble Capital Markets Head of Healthcare Banking Nathan Cali joins Maurizio to moderate a Q&A session. NobleCon 17 Complete Rebroadcast
|
Month: January 2021
Capstone Turbine (CPST) NobleCon17 Presentation Replay
|
|
|
Capstone Turbine (CPST) CEO Darren Jamison and CFO Eric Hencken at NobleCon17 – Noble Capital Markets 17th Annual Small & Microcap Investor Conference – January 2021. Following the formal presentation, Noble Capital Markets Senior Research Analyst Michael Heim joins Darren and Eric to moderate a Q&A session. NobleCon 17 Complete Rebroadcast
|
Adaptive Phage Therapeutics NobleCon17 Presentation Replay
|
|
|
Adaptive Phage Therapeutics CEO Greg Merril at NobleCon17 – Noble Capital Markets 17th Annual Small & Microcap Investor Conference – January 2021. Following the formal presentation, Noble Capital Markets Senior Research Analyst Michael Heim joins Greg to moderate a Q&A session. NobleCon 17 Complete Rebroadcast
|
Lineage Cell Therapeutics (LCTX) – CFO Left Reiterating Outperform Rating

Thursday, January 21, 2021
Lineage Cell Therapeutics (LCTX)
CFO Left, Reiterating Outperform Rating
Lineage Cell Therapeutics is a clinical-stage biotechnology company developing novel cell therapies for unmet medical needs. Lineage’s programs are based on its robust proprietary cell-based therapy platform and associated in-house development and manufacturing capabilities. With this platform Lineage develops and manufactures specialized, terminally differentiated human cells from its pluripotent and progenitor cell starting materials. These differentiated cells are developed to either replace or support cells that are dysfunctional or absent due to degenerative disease or traumatic injury or administered as a means of helping the body mount an effective immune response to cancer. Lineage’s clinical programs are in markets with billion dollar opportunities and include three allogeneic (“off-the-shelf”) product candidates: (i) OpRegen®, a retinal pigment epithelium transplant therapy in Phase 1/2a development for the treatment of dry age-related macular degeneration, a leading cause of blindness in the developed world; (ii) OPC1, an oligodendrocyte progenitor cell therapy in Phase 1/2a development for the treatment of acute spinal cord injuries; and (iii) VAC, an allogeneic dendritic cell therapy platform for immuno-oncology and infectious disease, currently in clinical development for the treatment of non-small cell lung cancer. For more information, please visit www.lineagecell.com or follow the Company on Twitter @LineageCell.
Ahu Demir, Ph. D., Biotechnology Research Analyst, Noble Capital Markets, Inc.
Refer to the full report for the price target, fundamental analysis, and rating.
CFO left. Lineage announced that Brandi Roberts, Chief Financial Officer, is leaving the company, effective January 20th, 2020 following her resignation on January 15th, 2021. Reasons for her leave were personal. She will be taking a CFO position at a private company, Longboard Pharmaceuticals.
CEO will serve as an interim CFO. The company has initiated efforts to find another CFO. Ms. Roberts also agreed to provide consulting services to Lineage for an orderly and smooth transition to the new CFO. Chief Executive Officer Brian Culley will serve as Interim Chief Financial Officer until the new CFO is appointed …
This Company Sponsored Research is provided by Noble Capital Markets, Inc., a FINRA and S.E.C. registered broker-dealer (B/D).
*Analyst certification and important disclosures included in the full report. NOTE: investment decisions should not be based upon the content of this research summary. Proper due diligence is required before making any investment decision.
Vista Gold (VGZ) NobleCon17 Presentation Replay
|
|
|
Vista Gold (VGZ) CEO Frederick Earnest at NobleCon17 – Noble Capital Markets 17th Annual Small & Microcap Investor Conference – January 2021. Following the formal presentation, Noble Capital Markets Senior Research Analyst Mark Reichman joins Frederick to moderate a Q&A session. NobleCon 17 Complete Rebroadcast
|
VolitionRx (VNRX) NobleCon17 Presentation Replay
|
|
|
VolitionRx (VNRX) CFO Scott Powell and CEO Cameron Reynolds at NobleCon17 – Noble Capital Markets 17th Annual Small & Microcap Investor Conference – January 2021. Following the formal presentation, Noble Capital Markets Senior Research Analyst Ahu Demir, PhD joins Scott and Cameron to moderate a Q&A session. NobleCon 17 Complete Rebroadcast
|
OS Therapies NobleCon17 Presentation Replay
|
|
|
OS Therapies CEO Paul Romness and Chief Medical & Scientific Officer Robert Petit at NobleCon17 – Noble Capital Markets 17th Annual Small & Microcap Investor Conference – January 2021. Following the formal presentation, Noble Capital Markets Senior Research Analyst Ahu Demir, PhD joins Paul and Robert to moderate a Q&A session. NobleCon 17 Complete Rebroadcast
|
Lineage Cell Therapeutics (LCTX) NobleCon17 Presentation Replay
|
|
|
Lineage Cell Therapeutics (LCTX) CEO Brian Culley at NobleCon17 – Noble Capital Markets 17th Annual Small & Microcap Investor Conference – January 2021. Following the formal presentation, Noble Capital Markets Senior Research Analyst Ahu Demir, PhD joins Brian to moderate a Q&A session. NobleCon 17 Complete Rebroadcast
|
Voyager Digital (VYGVF) NobleCon17 Presentation Replay
|
|
|
Voyager Digital (VYGVF) CEO Steve Ehrlich at NobleCon17 – Noble Capital Markets 17th Annual Small & Microcap Investor Conference – January 2021. Following the formal presentation, Noble Capital Markets Senior Research Analyst Joe Gomes joins Steve to moderate a Q&A session. NobleCon 17 Complete Rebroadcast
|
Xenetic Biosciences (XBIO) NobleCon17 Presentation Replay
|
|
|
Xenetic Biosciences (XBIO) CEO Jeffrey Eisenberg at NobleCon17 – Noble Capital Markets 17th Annual Small & Microcap Investor Conference – January 2021. Following the formal presentation, Noble Capital Markets Senior Research Analyst Ahu Demir, PhD joins Jeffrey to moderate a Q&A session. NobleCon 17 Complete Rebroadcast
|
Release – Kiromic (KRBP) – Announces the Completion of its GMP Manufacturing Facility in Houston Texas

Kiromic Announces the Completion of its GMP Manufacturing Facility in Houston, Texas, to Support the Manufacturing of the First In-Human Allogenic CAR-T Trial
Kiromic BioPharma, Inc. (Nasdaq: KRBP), an immuno-oncology target discovery and gene-editing company, with a proprietary artificial intelligence neural network platform (Diamond AI) to develop novel oncology therapeutics, has announced the completion and certification of its GMP facility in Houston, Texas:
- GMP facility construction is complete and certified to meet all FDA required regulatory guidelines.
- GMP facility is ready to support our upcoming first in-human, off-the-shelf, allogenic CAR-T trial. The CAR-T ovarian cancer trials will have these targets: chPD1 Gamma-Delta and anti-ISOMSLN.
- Gamma-Delta-T cell (GDT cell) GMP manufacturing test batch optimization and qualification studies are proceeding as scheduled.
“The in-house capability to manufacture allogenic, off-the-shelf CAR products removes a layer of complexity in the workflow, which we believe will greatly enhance our ability to move swiftly through our CAR-T trials,” says Dr. Maurizio Chiriva-Internati, PhD, CEO of Kiromic BioPharma.
“The key features of the facility have been completed, clearing the path for the production of our off-the-shelf Gamma-Delta-T cells, a novel approach to CAR-T cell therapy, which will be evaluated in the upcoming clinical trials,” says David Aguilar, PhD, Head of CMC Manufacturing of Kiromic BioPharma.
“Thanks to the hard work of our scientists, contractor engineers, suppliers, and the third party certifiers, our Houston Facility is completing the final steps needed for launching the next-generation of allogenic, off-the-shelf CAR-T,” says Mr. Tony Tontat, CFO, COO of Kiromic BioPharma.
(chPD1: Chimeric PD1; ISOMSLN: Iso-mesothelin; CAR: Chimeric Antigen Receptors)
About Allogenic Gamma-Delta-T cells
GDT cells are a small fraction of blood lymphocytes, but they are the predominant T cell type in epithelia, where they patrol the barrier between the body and the outside world, with their potent multi-anti-pathogen abilities. Compared with alpha-beta-T cells (ABT), currently used in CAR therapies, GDT cells are more efficient in killing tumor cells and more resistant to mechanisms by which solid tumors escape from the immune system.
Furthermore, unlike ABT cells, GDT cells manufacturing does not require the patient’s blood, but can be produced using the blood of healthy donors, a process called allogenic cell transfer. This is possible because, while ABT cells from another individual will attack a patient’s normal organs, GDT cells will only target the tumor target for which they were engineered, leaving non-tumoral cells untouched.
The workflow of CAR-T therapy is as follows:
- The GDT cells are grown and purified from the blood of healthy individuals.
- The GDT cells are expanded with a proprietary process.
- The GDT cells are then genetically modified to target tumor antigens discovered by Kiromic’s Diamond AI technology, resulting in the next generation of CAR-T cell therapy for solid malignancies.
About Kiromic
Kiromic BioPharma, Inc. is an immuno-oncology biopharmaceutical company focused on discovering, developing, and commercializing novel immuno-oncology applications through its robust product pipeline. The pipeline development is leveraged through the Company’s proprietary target discovery engine called “DIAMOND.” Kiromic’s DIAMOND is essentially big data science meeting target identification, dramatically compressing the man-years and the millions of drug development dollars needed to develop a live drug. The Company’s headquarters are located in Houston, TX adjacent to the world’s largest medical center and the MD Anderson Cancer Center.
For more information, please visit Kiromic’s website at:
Forward-Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties. We make such forward-looking statements pursuant to the safe harbor provisions of the U.S. Private Securities Litigation Reform Act, Section 21E of the Securities Exchange Act of 1934, as amended, and other federal securities laws. All statements other than statements of historical facts are forward-looking statements. These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Forward-looking statements include, but are not limited to, statements about our intentions, projections, assessments, or expectations regarding items such as the following:
- our goals and strategies
- research, development, and regulatory activities
- FDA authorization timeline for clinical trial initiation
- clinical trial enrollment or participation by clinical sites
- facility manufacturing capabilities for clinical trial support
- expectations related to gamma delta CAR therapy
- performance and success of clinical trials
- our future business development, financial condition, and results of operations
- expected changes in our revenue, costs, or expenditures
- growth of and competition trends in our industry
- our expectations regarding demand for, and market acceptance of, our products
- our expectations regarding our relationships with investors, institutional funding partners and other parties we collaborate with
- fluctuations in general economic and business conditions in the markets in which we operate; including those fluctuations caused by COVID-19
- relevant government policies and regulations relating to our industry.
In some cases, you can identify forward-looking statements by terms such as “may,” “could,” “will,” “should,” “would,” “expect,” “plan,” “intend,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “project” or “continue” or the negative of these terms or other comparable terminology. These statements are only predictions. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, which are, in some cases, beyond our control and which could materially affect results. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under the heading “Risk Factors” included in our Registration Statement on Form S-1 (file no. 333-238153), originally filed with the Securities and Exchange Commission (SEC) on May 11, 2020, as amended, and elsewhere in this press release. If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those implied or projected by the forward-looking statements. No forward-looking statement is a guarantee of future performance.
The forward-looking statements made in this press release relate only to events or information as of the date on which the statements are made in this press release. Except as expressly required by the federal securities laws, there is no undertaking to publicly update or revise any forward-looking statements, whether as a result of new information, future events, changed circumstances or any other reason. You are advised, however, to review any further disclosures we make on related subjects in our Forms 10-Q, 8-K and other reports filed with the SEC.
Tony Tontat
Chief Financial Officer
(844) 539 – 2873
ttontat@kiromic.com
SOURCE: Kiromic
Release – CoreCivic (CXW) – Establishes New Reentry-Focused Leadership Role

CoreCivic Establishes New Reentry-Focused Leadership Role
Daren Swenson will serve as Vice President, Reentry Partnerships and Innovation
BRENTWOOD, Tenn. – January 19, 2021 – CoreCivic, Inc. (NYSE: CXW) announced today the creation of a new leadership role dedicated to building on the company’s longstanding efforts to provide innovative, high-quality reentry programming to help tackle America’s recidivism crisis. Daren Swenson will serve as the company’s first Vice President, Reentry Partnerships and Innovation. He previously led CoreCivic Community, which provides residential and nonresidential services to help justice-involved individuals obtain employment, housing, healthcare, mental health and addiction treatment, and family reunification, as they successfully reintegrate into their communities.
“CoreCivic has a strong track record of leadership in the fight against recidivism, including helping those in our care learn the life and vocational skills they need,” said Damon T. Hininger, President and CEO. “From making unprecedented commitments to reentry programming in 2014 to launching our effort to support reentry-friendly public policies in 2017, the creation of this new leadership position is a critical next step in our efforts to take on one of the biggest challenges facing our country.”
In his new role, Swenson will serve as CoreCivic’s top advocate and practitioner for reentry. He will build on the company’s ongoing efforts to cultivate meaningful partnerships with academics, issue experts, policymakers and other organizations dedicated to effective reentry solutions and recidivism-reducing outcomes. He will also work to operationalize innovative programs and best practices learned from these partnerships, as well as share the lessons learned through CoreCivic’s extensive efforts to promote successful reentry programs and policies.
“Having served in this field for nearly 30 years, it’s the greatest opportunity of my career to take on a role dedicated to reentry partnerships and innovation,” said Swenson. “I see the difference our teachers, chaplains, counselors, case managers, officers, monitors and so many others make every day and know that there’s even more we can do to positively impact people as they seek to rejoin their families and communities successfully.”
Swenson holds a bachelor’s degree in psychology and sociology from North Dakota State University, and he has served in numerous roles in secure and community corrections, including as warden of facilities in Oklahoma, Minnesota and Arizona.
“Daren has a broad mandate to continue developing deep relationships with people and organizations dedicated to reducing recidivism and to bring innovative approaches into our facilities,” said Hininger. “We know that the challenges justice-involved individuals face cannot be solved by one organization alone, and we look forward to working closely with partners dedicated to providing the best in reentry.”
CoreCivic is a diversified, government-solutions company with the scale and experience needed to solve tough government challenges in flexible, cost-effective ways. We provide a broad range of solutions to government partners that serve the public good through high-quality corrections and detention management, a network of residential and non-residential alternatives to incarceration to help address America’s recidivism crisis, and government real estate solutions. We are the nation’s largest owner of partnership correctional, detention and residential reentry facilities, and believe we are the largest private owner of real estate used by U.S. government agencies. We have been a flexible and dependable partner for government for more than 35 years. Our employees are driven by a deep sense of service, high standards of professionalism and a responsibility to help government better the public good. Learn more at www.corecivic.com.
SOURCE: CoreCivic
Release – Palladium One Mining (NKORF)(PDM:CA) – Additional Massive Magmatic Sulphide Intersections at Tyko

Palladium One Additional Massive Magmatic Sulphide Intersections, up to 9.9% Ni_Eq (218 lbs/tonne) over 3.8 Meters at Tyko
January 19, 2021 – Toronto, Ontario – Final results from the 2020 Tyko drill program include massive magmatic sulphides grading up to 9.9% Ni_Eq* (218 pounds per tonne) over 3.8 Meters (8.1% Ni, 2.9% Cu, 1.3/t PGE), starting at less than 9 meters true-depth, located at the Smoke Lake target of the Tyko Ni-Cu-PGE Project said Palladium One Mining (“Palladium One” or the “Company”) (TSXV: PDM, FRA: 7N11, OTC: NKORF) today. The intercept is within a broader interval that returned 6.1% Ni_Eq over 7.5 Meters (135 pounds per tonne) (4.5% Ni, 2.9% Cu, 1.0g/t PGE) from 5.3 meters down hole.
These results are in addition to previously announced results of 8.7% Ni_Eq* (193 pounds per tonne) over 3.8 Meters (6.6% Ni, 3.7% Cu, 1.5g/t PGE) (see press release January 5, 2021) and 7.5% Ni_Eq* (164 pounds per tonne) over 4.2 Meters (5.8% Ni, 2.7% Cu, 1.3/t PGE) (see press release January 12, 2021).
“Smoke Lake continues to deliver extraordinarily high-grade intercepts. The highest to date being 9.9% Ni_Eq over 3.8 meters, within a broader intercept of 6.1% Ni_Eq over 7.5 meters! An extremely high-value, near surface resource appears within our grasp at Smoke Lake.
The massive sulphide mineralization discoveries, combined with historic high-grade drill results 17-km to the west, provide significant encouragement for additional discoveries, especially given Tyko is incredibly underexplored. The Tyko project covers over 20,000 hectares, which includes the 7,000 hectare mafic-ultramafic Bulldozer intrusion, which has seen virtually no geological mapping nor exploration” said Derrick Weyrauch, President and CEO.
Key Highlights:
- Hole TK-20-023 returned 6.1% Ni_Eq over 7.5 meters (4.5% Ni, 2.9% Cu, 1.0g/t PGE) from 5.3 meters down hole.
- Including 9.9% Ni_Eq over 3.8 meters (8.1% Ni, 2.9% Cu, 1.3g/t PGE).
-
- Including 11.8% Ni_Eq over 0.6 meters (9.6% Ni, 3.7% Cu, 1.5g/t PGE).
- All 13 holes drilled at Smoke Lake intersected magmatic sulphides.
- Multiple massive sulphide intercepts up to 4 metres were encountered.
- A magmatic sulphide mineralized strike length of 270 meters has been defined by drilling and the deepest intercept to date has a true depth of only 100 meters.
- Mineralization remains open to the northwest and down dip.
Hole TK-20-025, returned 6.3% Ni_Eq over 3.2 meters (4.4% Ni, 3.6% Cu, 0.9g/t PGE) from 36.6 meters down hole.
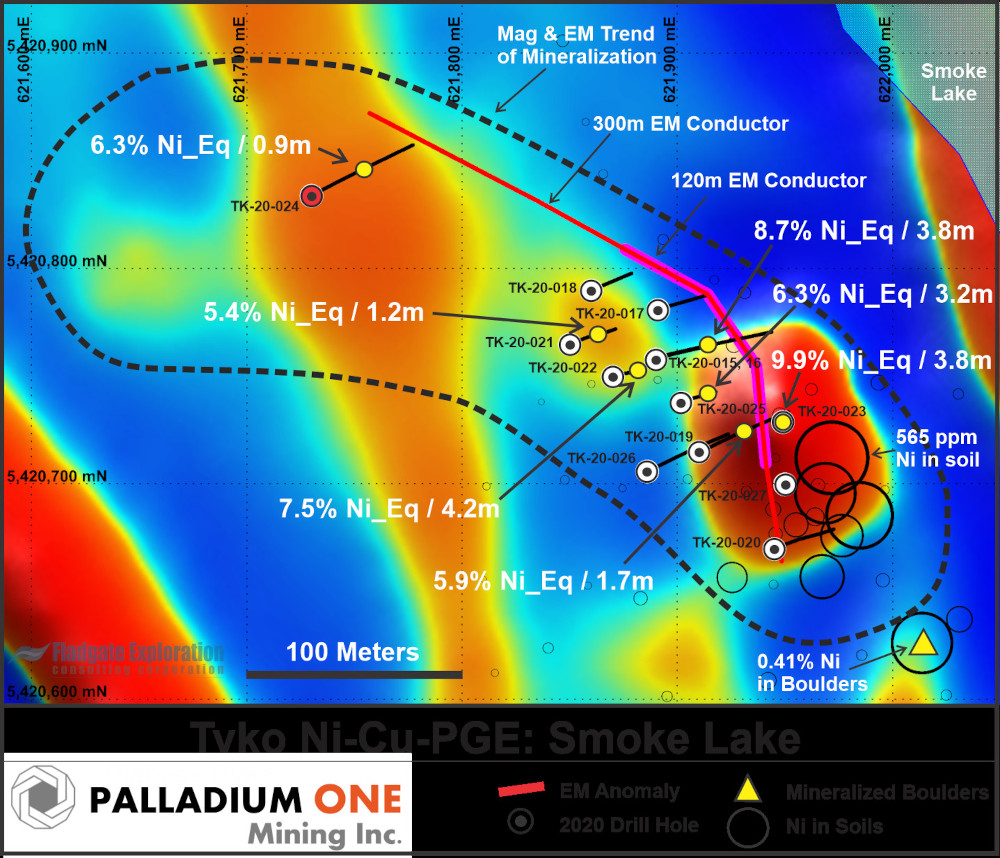
The 2020 Tyko drill program consisted of 14 drill holes totalling 1,123 meters, 13 holes were drilled into the Smoke Lake electromagnetic (“EM”) anomaly. This program was the first to drill test the Smoke Lake EM anomaly (see press release January 21, 2020, November 18, 2020, December 7, 2020, January 5, 2021, January 12, 2021). High-resolution drone-based magnetic and ground-based horizontal loop EM surveys, undertaken shortly before drilling, refined the anomaly resulting in the successful discovery of massive magmatic sulphides. The final hole of the program (TK-20- 028) tested a separate magnetic anomaly which intersected mafic-ultramafic rocks with anomalous nickel which are interpreted to be related to the Smoke lake mineralization.
A bore hole EM survey is currently underway which will further delineate the Smoke Lake massive sulphide body. Palladium One Mining Inc. Suite 550 – 800 West Pender St. | Vancouver, BC | Canada V6C 2V6 info@palladiumoneinc.com Drilling to date indicates a mineralized ultramafic body at surface, transitioning to massive sulphides which dip shallowly (~32°) to the southwest. The massive sulphides occur as a consistent sheet with a possible fault near its base which could be controlling their emplacement in tonalite.
The lithologies at Smoke Lake closely resemble those found at both the Tyko and RJ showings, located 17-kilometers to the west, which returned up to 1.06% Ni and 0.35% Cu over 6.22 m including 4.71% Ni and 0.82% Cu over 0.87 m in hole TK-16-010 (see press release June 8, 2016).
Table 1: Tyko 2020 Drill Results from the Smoke Lake Discovery
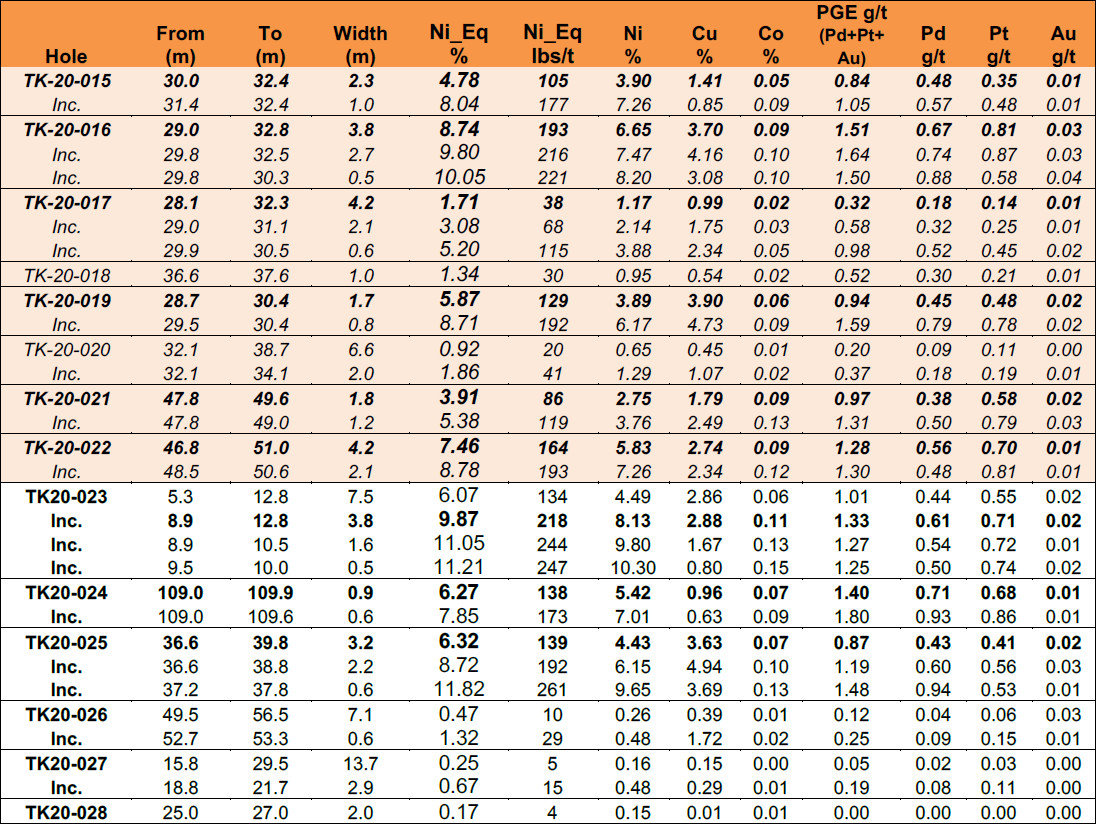
(1) Reported widths are “drilled widths” not true widths.
(2) Shaded results are previously released, see press release January 5, 2020, January 12, 2021
(3) TK-20-028 tested a different target on the Tyko Property.
Figure 1. Massive magmatic sulphide intersection in hole TK-20-023.

Figure 2. Closeup of massive magmatic sulphide in hole TK-20-023.

Figure 3. High grade intersection from hole TK-20-025 which returned 11.8% Ni_Eq over 0.6 meters (9.6% Ni, 3.7% Cu, 1.5g/t PGE) from 37.2 to 37.8m

Figure 4. Plan map of the Smoke Lake area with 1st Vertical Mag as the background showing soil samples, as well as the axial traces of the two closely spaced ground based horizontal loop EM anomalies, and 2020 drill holes.
*Nickel Equivalent (“Ni_Eq”)
Nickel equivalent is calculated using US$1,100 per ounce for palladium, US$950 per ounce for platinum, US$1,300 per ounce for gold, US$6,614 per tonne (US$3.00 per pound) for copper, US$15,432 per tonne (US$7.00 per pound) for nickel and US$30,865 per tonne (US$14 per pound) for Cobalt. This calculation is consistent with the commodity prices used in the Company’s September 2019 NI 43-101 Kaukua resource estimate.
About Tyko Ni-Cu-PGE Project
The Tyko Ni-Cu-PGE Project, is located approximately 65 kilometers northeast of Marathon Ontario, Canada. Tyko is an early stage, high sulphide tenor, nickel focused project with the most recent drill hole intercepts returning up to 9.9% Ni_Eq over 3.8 meters (8.1% Ni, 2.9% Cu, 1.3g/t PGE) in hole TK-20-023.
Qualified Person
The technical information in this release has been reviewed and verified by Neil Pettigrew, M.Sc., P. Geo., Vice President of Exploration and a director of the Company and the Qualified Person as defined by National Instrument 43- 101.
About Palladium One
Palladium One Mining Inc. is an exploration company targeting district scale, platinum-group-element (PGE)-coppernickel deposits in Finland and Canada. Its flagship project is the Läntinen Koillismaa or LK Project, a palladiumdominant platinum group element-copper-nickel project in north-central Finland, ranked by the Fraser Institute as one of the world’s top countries for mineral exploration and development. Exploration at LK is focused on targeting disseminated sulfides along 38 kilometers of favorable basal contact and building on an established NI 43-101 open pit resource.
ON BEHALF OF THE BOARD
“Derrick Weyrauch”
President & CEO, Director
For further information contact:
Derrick Weyrauch, President & CEO
Email:
info@palladiumoneinc.com
Neither the TSX Venture Exchange nor its Market Regulator (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
This press release is not an offer or a solicitation of an offer of securities for sale in the United States of America. The common shares of Palladium One Mining Inc. have not been and will not be registered under the U.S. Securities Act of 1933, as amended, and may not be offered or sold in the United States absent registration or an applicable exemption from registration.
Information set forth in this press release may contain forward-looking statements. Forward-looking statements are statements that relate to future, not past events. In this context, forward-looking statements often address a company’s expected future business and financial performance, and often contain words such as “anticipate”, “believe”, “plan”, “estimate”, “expect”, and “intend”, statements that an action or event “may”, “might”, “could”, “should”, or “will” be taken or occur, or other similar expressions. By their nature, forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements, or other future events, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Such factors include, among others, risks associated with project development; the need for additional financing; operational risks associated with mining and mineral processing; fluctuations in palladium and other commodity prices; title matters; environmental liability claims and insurance; reliance on key personnel; the absence of dividends; competition; dilution; the volatility of our common share price and volume; and tax consequences to Canadian and U.S. Shareholders. Forward-looking statements are made based on management’s beliefs, estimates and opinions on the date that statements are made and the Company undertakes no obligation to update forward-looking statements if these beliefs, estimates and opinions or other circumstances should change. Investors are cautioned against attributing undue certainty to forward-looking statements.
Source: Palladium One Mining Inc.









