Palladium One Step-Out Drill Hole Intersects Shallow, High-Grade Mineralization at Murtolampi Zone, Finland
November 16, 2020 – Toronto, Ontario – Starting at only 11 m down hole, hole LK20-026 at the Murtolampi Zone, intersected high-grade open pit potential mineralization returning 13 m at 3.4 g/t palladium equivalent (Pd_Eq)* within 79 m at 2.0 g/t Pd_Eq (Figure 1, 2 and 3). said Palladium One Mining Inc. (“Palladium One” or the “Company”) (TSXV: PDM, FRA: 7N11, OTC: NKORF) today.
Key highlights:
- Shallow high-grade results suggest potential for a low-cost satellite open pit at Murtolampi, which is close to the existing Kaukua deposit, located 2 km to the south.
- Murtolampi remains open for expansion both laterally and at depth.
- A core, near surface interval of 13 m at 3.4 g/t Pd_Eq starting 66 m down hole in hole LK20-026 returned 79 m at 2.0 g/t Pd_Eq starting 11.0 m down hole.
- Hole LK20-026 is located 50 m southwest of Hole LK20-012 which returned 20 m at 2.3 g/t Pd_Eq, starting 29 m down hole within 87 m @ 1.4 g/t Pd_Eq starting 5.8 m downhole (see news release dated August 25, 2020)
- Hole LK20-026 is also located 550 m northeast of hole LK20-024 which returned a core interval of 3 m at 1.4 g/t Pd_Eq. within 21 m at 0.85 g/t Pd_Eq.
- The mineralized peridotite at Murtolampi has now been defined over a 600 m strike length.
“The at surface mineralization at Murtolampi continues to advance a potentially valuable satellite pit to the Kaukua deposit which is located only 2 km to the south.” said Derrick Weyrauch, President and Chief Executive Officer. “Hole LK20-026 is by far the highest-grade hole drilled to date at Murtolampi, we look forward to additional results from this zone in the Phase II drill program.”
Palladium One has confirmed PGE-Ni-Cu mineralization over 600 m of strike length at the Murtolampi zone and the zone remains open for expansion laterally and at depth (Figure 1, 2 and 3).
The Murtolampi zone hosts both mineralized (holes LK20-12, 024 & 026) and unmineralized (hole (LK20-025) peridotite. Mineralized peridotite contains blebby Cu-Ni sulphides and strong PGE mineralization and produces a modest Induced Polarization (IP) chargeability anomaly. Unmineralized peridotite is Ni bearing and Cu-PGE-poor while containing fine grain disseminated pyrite which produces strong IP chargeability anomalies. These two peridotite phases are in contact with one another at Murtolampi, making distinctions between the IP anomalies difficult. The overall geometry of the Murtolampi zone is yet to be defined, additional holes including undercut and scissor holes are planned in the Phase II drill program to better define the mineralization peridotite body.
Phase II Drill Program
Based on its discovery success this year, the Company has launched a 17,500 m phase II diamond drill program (see news release November 10, 2020) which will be focused on the Kaukua South and Murtolampi zones.
Figure 1
This figure shows the greater Kaukua Area, the NI 43-101 compliant Kaukua Open Pit resource, Murtolampi and Kaukua South zones. Select resumed Phase I drill holes labelled in red.
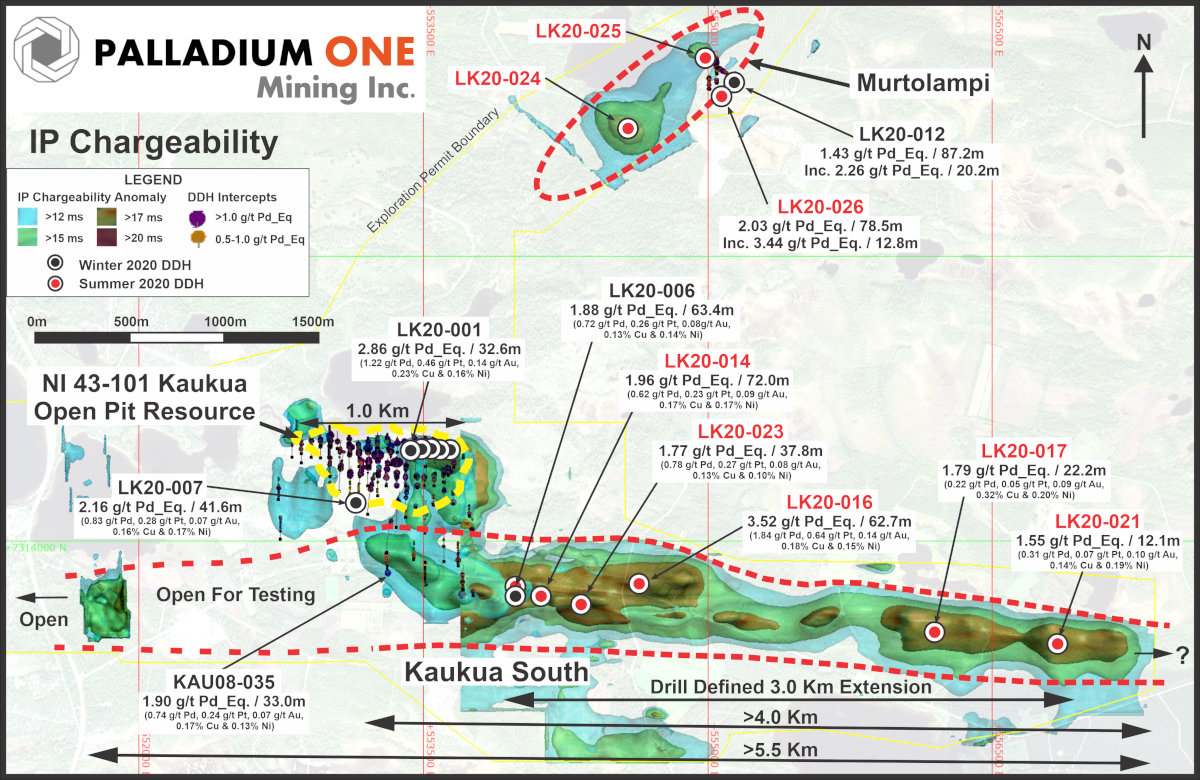
Figure 2
Murtolampi Cross section showing hole LK20-012 looking southwest, showing IP Chargeability isoshells and Pd_Eq grade.
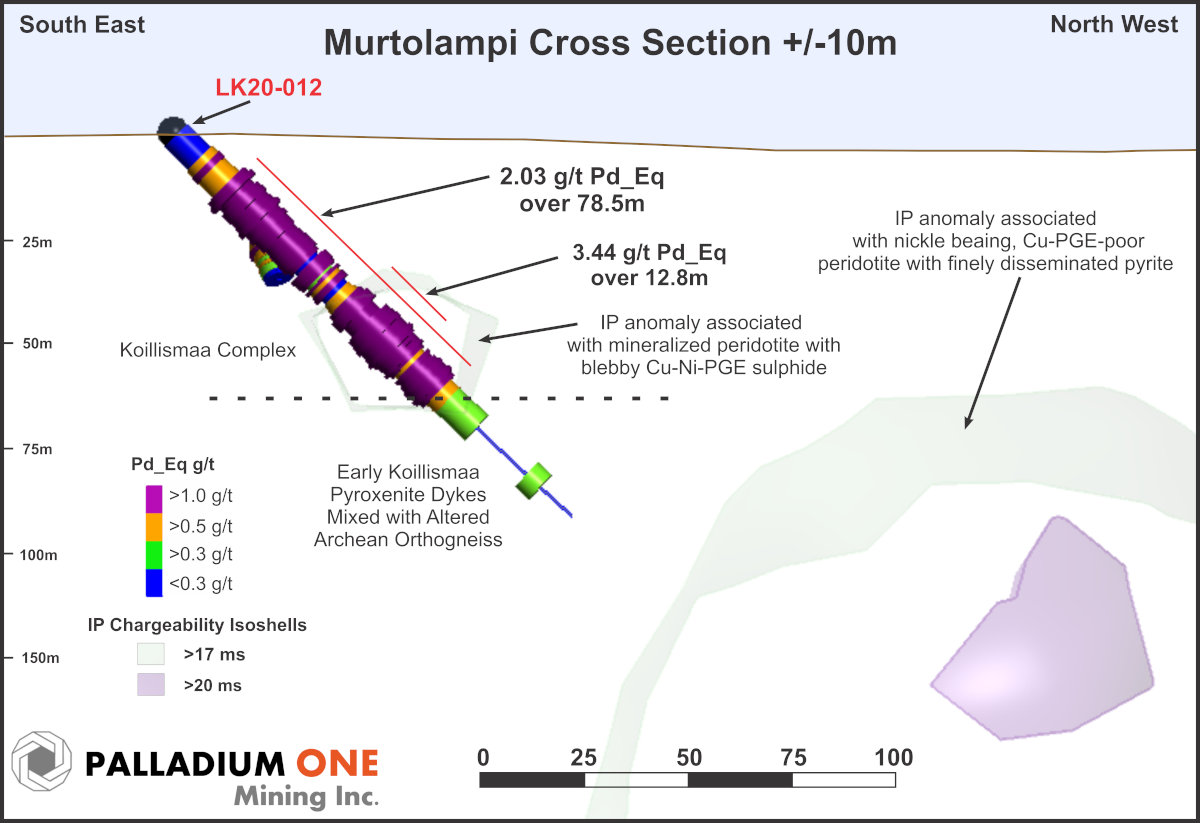
Figure 3
Murtolampi Long section looking northwest, showing IP Chargeability isoshells and Pd_Eq grade, resumed Phase I drill holes labelled in red.
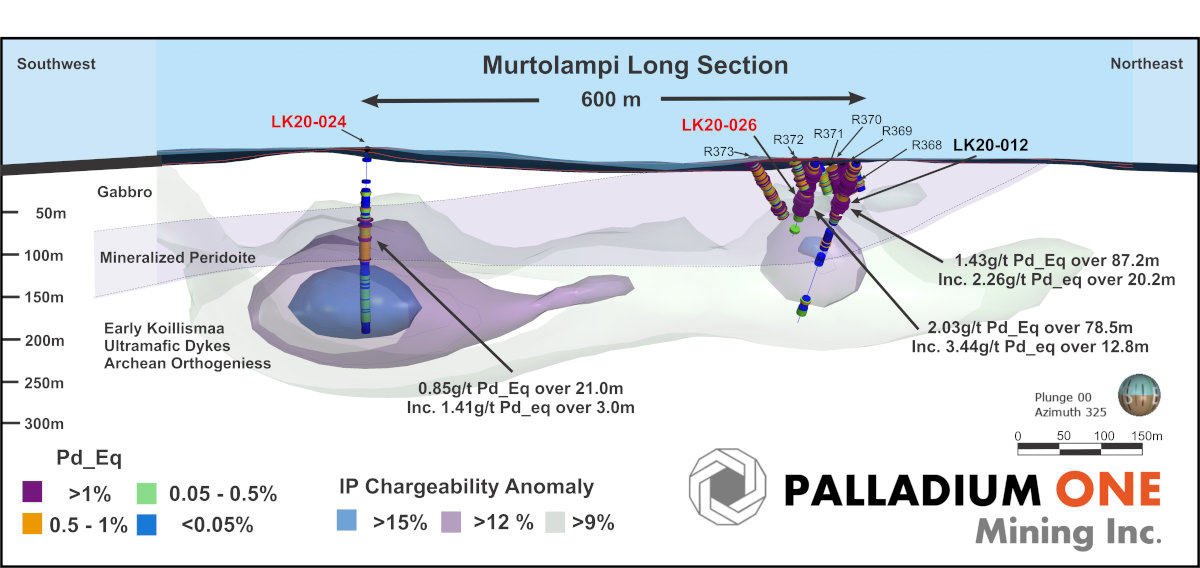
Table 1: Resumed Phase 1 Drill Results from Murtolampi Zone
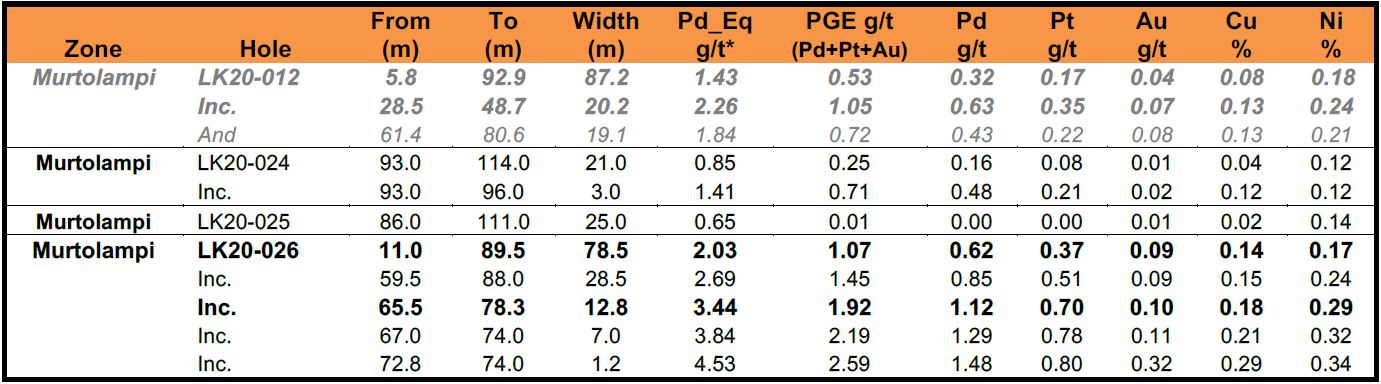
* Reported widths are “drilled widths” not true widths.
** Grey Italicised values are previously released (see press release August 25, 2020)
*Palladium Equivalent
Palladium equivalent is calculated using US$1,100 per ounce for palladium, US$950 per ounce for platinum, US$1,300 per ounce for gold, US$6,614 per tonne for copper, and US$15,4332 per tonne for nickel. This calculation is consistent with the calculation in the Company’s September 2019 NI 43-101 Kaukua resource estimate.
QA/QC
The Phase I drilling program was carried out under the supervision of Neil Pettigrew, M.Sc., P. Geo., Vice President of Exploration and a director of the Company.
Drill core samples were split using a rock saw by Company staff, with half retained in the core box and stored indoors in a secure facility, in Taivalkoski, Finland. The drill core samples were transported by courier from the Company’s core handling facility in Taivalkoski, Finland, to ALS Global (“ALS”) laboratory in Outokumpu, Finland. ALS, is an accredited lab and are ISO compliant (ISO 9001:2008, ISO/IEC 17025:2005). PGE analysis was performed using a 30 grams fire assay with an ICP-MS or ICP-AES finish. Multi-element analyses, including copper and nickel were analysed by four acid digestion using 0.25 grams with an ICP-AES finish.
Certified standards, blanks and crushed duplicates are placed in the sample stream at a rate of one QA/QC sample per 10 core samples. Results are analyzed for acceptance at the time of import. All standards associated with the results in this press release were determined to be acceptable within the defined limits of the standard used
Qualified Person
The technical information in this release has been reviewed and verified by Neil Pettigrew, M.Sc., P. Geo., Vice President of Exploration and a director of the Company and the Qualified Person as defined by National Instrument 43- 101.
About Palladium One
Palladium One Mining Inc. is an exploration company targeting district scale, platinum-group-element (PGE)-copper-nickel deposits in Finland and Canada. Its flagship project is the Läntinen Koillismaa or LK Project, a palladium-dominant platinum group element-copper-nickel project in north-central Finland, ranked by the Fraser Institute as one of the world’s top countries for mineral exploration and development. Exploration at LK is focused on targeting disseminated sulfides along 38 kilometers of favorable basal contact and building on an established NI 43-101 open pit resource.
ON BEHALF OF THE BOARD
“Derrick Weyrauch”
President & CEO, Director
For further information contact:
Derrick Weyrauch, President & CEO
Email:
info@palladiumoneinc.com
Neither the TSX Venture Exchange nor its Market Regulator (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
This press release is not an offer or a solicitation of an offer of securities for sale in the United States of America. The common shares of Palladium One Mining Inc. have not been and will not be registered under the U.S. Securities Act of 1933, as amended, and may not be offered or sold in the United States absent registration or an applicable exemption from registration.
Information set forth in this press release may contain forward-looking statements. Forward-looking statements are statements that relate to future, not past events. In this context, forward-looking statements often address a company’s expected future business and financial performance, and often contain words such as “anticipate”, “believe”, “plan”, “estimate”, “expect”, and “intend”, statements that an action or event “may”, “might”, “could”, “should”, or “will” be taken or occur, or other similar expressions. By their nature, forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements, or other future events, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Such factors include, among others, risks associated with project development; the need for additional financing; operational risks associated with mining and mineral processing; fluctuations in palladium and other commodity prices; title matters; environmental liability claims and insurance; reliance on key personnel; the absence of dividends; competition; dilution; the volatility of our common share price and volume; and tax consequences to Canadian and U.S. Shareholders. Forward-looking statements are made based on management’s beliefs, estimates and opinions on the date that statements are made and the Company undertakes no obligation to update forward-looking statements if these beliefs, estimates and opinions or other circumstances should change. Investors are cautioned against attributing undue certainty to forward-looking statements.
Source: Palladium One Mining Inc.